At the risk of a grievous understatement, 2025 has brought significant shifts in US federal funding priorities
Clinical research, long dependent on support from the National Institutes of Health (NIH), the Food and Drug Administration (FDA) and CMS, is feeling the ripple effects. It’s still early but we are already observing that budget reallocation impacts and political scrutiny are having a chilling effect on innovation and diversity in trials.
Federal Funding Landscape: What’s Changing in 2025
- National Institutes of Health (NIH)
As of April 2025, the federal website that tracks clinical trials lists 45,000 active trials and the NIH is the largest funder. NIH is associated directly or indirectly with nearly every drug approved in recent years with NIH funding contributing to 354 out of 356 drugs (99.4%) approved between 2010 and 2019. In 2024, the overwhelming majority of the agency’s $47 billion budget supported research at 2,500+ scientific institutions.
As of June of this year, the NIH has ended 1,389 awards, with $820+ million in recent funding, and delayed sending funding to more than 1,000 additional projects with nearly $740 million. From January 2025 through April 2025, the NIH awarded $1.6 billion less compared with the same period in 2024, a decrease of one-fifth.
NIH “scoured grants for key words and phrases like ‘transgender,’ ‘misinformation,’ ‘vaccine hesitancy’ and ‘equity,’ ending those focused on certain topics or populations, according to a current NIH program officer, who asked not to be identified for fear of retribution.
In addition to cuts and delays, the current administration has proposed cutting NIH’s budget by $18 billion, a decrease of nearly 40 percent.
- Food and Drug Administration (FDA)
The current administration has made significant staff cuts across the entire FDA, including
the support staff for the facility inspection programs and the lab staff that tests samples from batches of Active Pharmaceutical Ingredients (APIs), the components that generate drugs’ impacts on disease.”
The job cuts reported in April 2025 – 1,900 layoffs and 1,200 early retirements – are expected to affect the regulation of the quality of generic drugs and reviews of new drug applications for near-generic biological products, such as biosimilars, and much more.
The proposed FDA budget for fiscal year 2026 is $6.8 billion, a 3.9 percent decrease from the $7.2 billion enacted in FY 2025. A reduced budget may slow down guidance, regulatory reviews, and innovation programs. At the same time, there may be pressure to accelerate approvals while simultaneously reducing staff and bureaucracy.
- Centers for Medicare and Medicaid Services (CMS)
The CMS has also felt the impact of recent federal leadership priorities and budget reductions, including coverage for trial-related procedures and reimbursement for decentralized models. There also has been pushback on 2025 digital health reimbursement policies centers, especially the “telehealth cliff” which would reinstate geographic restrictions, eliminate rural and home-based access, and remove the ability of federally qualified health centers (FQHCs) and rural health clinics (RHCs).
Changing approaches to Equity in Healthcare
The political resistance to health equity, diversity, equity and inclusion (DEI) initiatives, and community-based research is growing. State-level legal restrictions and policy shifts cut programs addressing social determinants of health (SdoH) and underserved populations are happening, with some states restricting DEI initiatives and other initiatives aiming to reverse policies designed to promote health equity. Since 2023, 18 states have passed legislation that restricts DEI initiatives, including elimination of DEI offices and staff, prohibition of mandatory diversity training, banning diversity statements, and forbidding the use of identity in hiring and admissions at public colleges and universities.
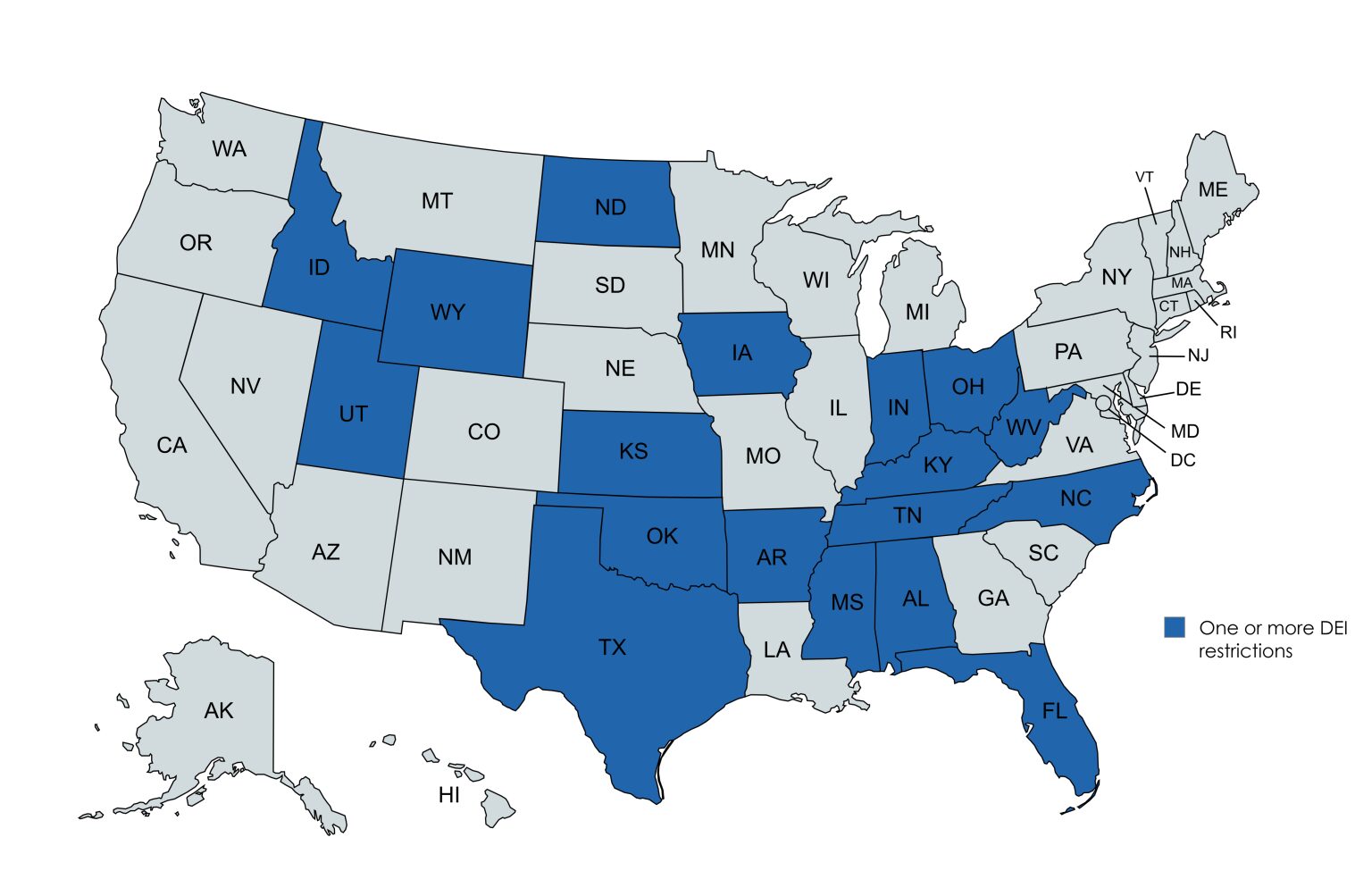
There are near- and long-term implications for representation in clinical trials, institutional review board (IRB) standards and inclusive recruitment. This year’s federal shifts are more than simply budget reallocation; they reflect deep tensions about the role of science in society. Sponsors, clinical research organizations (CROs) and academic teams must be both strategic and principled to weather this moment. The future of clinical research depends on its ability to innovate ethically and inclusively even when it’s not politically convenient.



