Know if your study's on track without digging through reports.
Visualize and analyze all your clinical data in real time—from enrollment tracking to adverse event alerts. Stop chasing spreadsheets and get the insights you need, when you need them.
Who It’s For

Academic Researchers
Stop wrangling data in spreadsheets. Get real-time visibility into enrollment, protocol compliance, and data quality without waiting days for reports.

Sponsors & CROs
Track study performance across your portfolio with real-time snapshots for database locks, SDV tracking, and query management. Analytics is part of your OpenClinica platform, not another reporting tool to manage.

Sites & Coordinators
Manage oversight across multiple clients and sites without jumping between systems. Give your teams centralized visibility into enrollment, data quality, and monitoring priorities—all in one place.
Benefits
Stop chasing spreadsheets
Replace slow, manual spreadsheets with real-time clinical data analysis. Get immediate results you can act on without waiting on statisticians.
Track enrollment automatically
Let Analytics maintain your study enrollment reports in real time. No more manual updates or out-of-date projections.
Instant adverse event alerts
Get notified when severe adverse events are reported, day or night, so your safety officers can respond immediately.
Faster data cleaning
Identify errors, outliers, and patterns early and often—before database lock—so you're not scrambling at the end.
More effective monitoring
Dashboards help you focus on the sites and data points that need attention most, reducing wasted monitoring visits.
Self-service reporting
Build point-and-click custom reports yourself, or use SQL directly if you prefer. Your choice. No IT tickets required.
Trusted By: 1500+ sponsors, CROs, and research sites worldwide

"OpenClinica's reporting and analytics capabilities gave us insights into patient demographics, treatment outcomes, and adverse events. The software's user-friendly interface streamlined and expedited the data entering process, decreasing errors and cutting down on data entry time."
Pharmaceutical Product Development and Research Intern

"Flexible and agile design with robust web services – OpenClinica delivers… The OpenClinica design tools make it easy for non-technical resources to make, test, and then deploy changes while maintaining compatibility with previous iterations."
Leading Clinical Laboratory Services Company
How it Works
Analytics gives you real-time visibility into your clinical data without the complexity of traditional Business Intelligence (BI) tools. Start with pre-built reports or build custom ones with point-and-click simplicity. Share dashboards with different stakeholders and automate alerts when critical events occur. With the transparent pricing and dedicated support small to midsize teams need.
Choose your starting point
Start with pre-built reports (enrollment, queries, SDV, AE tracking) or build custom reports using point-and-click tools or SQL
Create and share dashboards
Organize reports into interactive dashboards tailored for different stakeholders—data managers, CRAs, safety officers, or sponsors
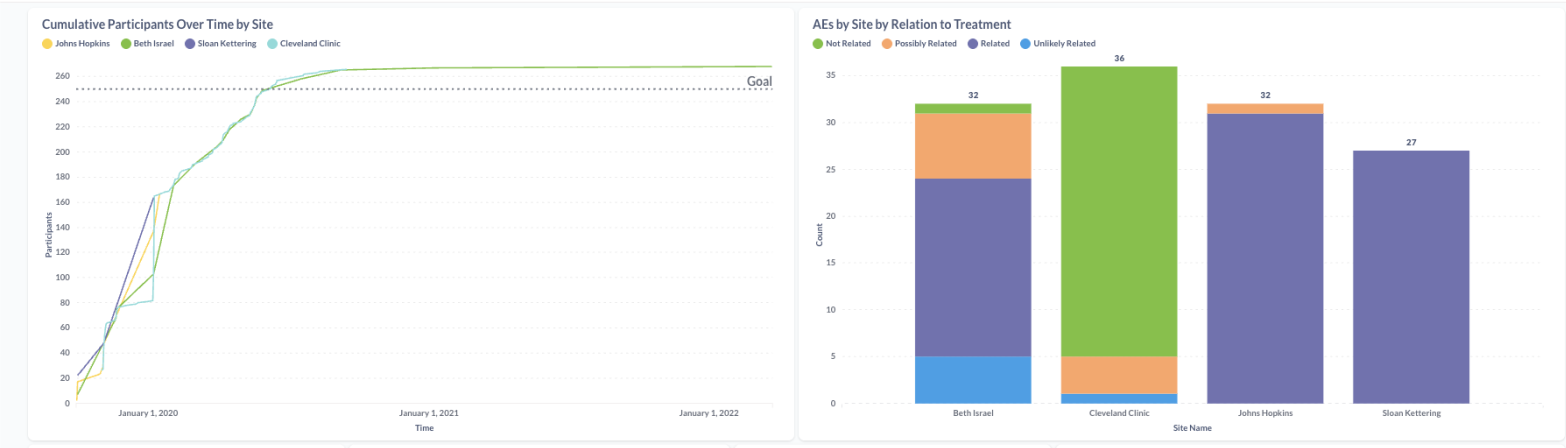
Explore and act
Use interactive visualizations to drill into your data, identify trends, and make faster decisions—with automated alerts when key events happen
Choose your starting point
Start with pre-built reports (enrollment, queries, SDV, AE tracking) or build custom reports using point-and-click tools or SQL

Choose your starting point
Create and share dashboards
Organize reports into interactive dashboards tailored for different stakeholders—data managers, CRAs, safety officers, or sponsors

Create and share dashboards
Explore and act
Use interactive visualizations to drill into your data, identify trends, and make faster decisions—with automated alerts when key events happen

Explore and act
Ready to stop chasing spreadsheets?
Ideal For
- Small to midsize sponsors and CROs
- Academic and investigator-initiated research
- Multi-site studies requiring centralized oversight
- Teams tired of manual Excel reporting
- Studies requiring real-time safety monitoring
- Data managers planning database locks
- CRAs needing focused monitoring dashboards
Features
Pre-Built Reports Include:
- CRF Completion by Event (completed vs. not completed by site)
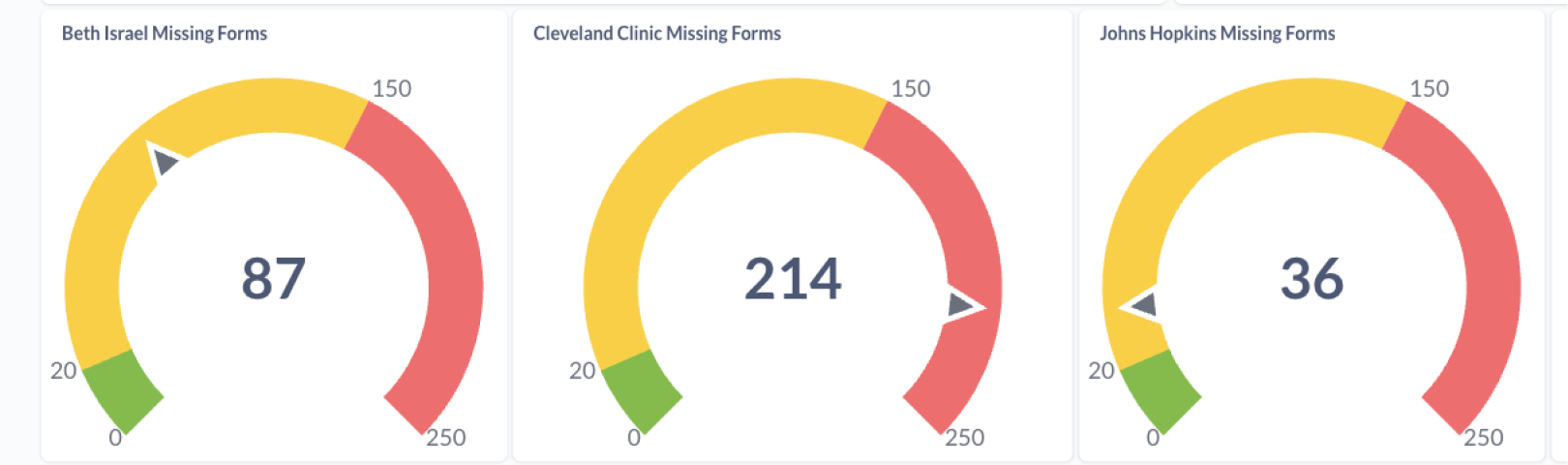
- Missing Forms (required forms not started in active events)
- Query Aging by Month, Site, and Study
- Source Data Verification (SDV) by Event and Site
- Adverse event (AE) and serious adverse event (SAE) alerts
Know more
- Enrollment tracking and projections
- Adverse event (AE) and serious adverse event (SAE) alerts
Core Capabilities:
- Point-and-click report builder OR direct SQL access
- Unlimited custom reports and interactive dashboards
- Real-time data from OpenClinica EDC
- Access to clinical data, ePRO data, query data, monitoring data, audit logs
- Interactive visualizations with drill-down from cohorts to individual patients
- One-click exports to Excel, PDF, and other formats
- Fine-grained role-based permissions
- Automated alerts and scheduled email reports
- Share reports with team members, sites, and sponsors
- Customizable dashboards for different stakeholders
- Built on well-documented data warehouse
- Training and support included
- Modular design—works with OpenClinica EDC
- Transparent, predictable pricing
Ready to stop chasing spreadsheets?
Tell us what questions you need to answer about your studies. We’ll explore how Analytics gives you real-time visibility without the complexity of traditional reporting tools.
Request a Demo
"*" indicates required fields