Consent people truly understand.
Make informed consent easier, more engaging, and fully compliant. Meet participants wherever they are with media-rich content that works on any device. No downloads. No IT headaches. Just a connected experience that integrates directly with your study.
Who It’s For

Academic Researchers
Simplify IRB compliance and improve participant comprehension without adding complexity to your workflow.

Sponsors & CROs
Streamline consent across decentralized, hybrid, and in-person trials with automated re-consenting workflows that keep studies on track.

Sites & Coordinators
Eliminate paper bottlenecks and reduce time spent chasing signatures. See consent status in real-time, right inside your study platform.
Benefits
Better engagement from the start
Use images, audio, and video to explain complex concepts clearly. Participants can review at their own pace, in their own language.
Verify true comprehension
Embedded questions confirm understanding before participants sign—reducing protocol deviations and improving data quality.
Zero friction for participants
Nothing to download or install. Works on any device participants already own. Automated reminders keep the process moving.
Built for real-world trials
Purpose-built for decentralized, remote, in-person, and hybrid settings. Supports single or multiple signatures, multilingual populations, and automated LAR (Legally Authorized Representative) workflows.
Direct integration with your study
See consent status in real-time inside OpenClinica EDC. No more logging in and out of different systems or hunting for documents.
Automated re-consenting workflows
When protocols change, re-consenting workflows trigger automatically—keeping studies compliant without manual tracking.
Trusted by 1500+ sponsors, CROs, and research sites worldwide

"OpenClinica Consent is a very well designed and created system, offering noticeably simple ways to improve the clinical trial process! The system is very intuitive and easy to implement in all trials. A big plus is the ease of authentication, which will certainly facilitate training for sites and patients, the ability to add photos or videos to the form, and the availability of a PDF version for the patient."
Andreas Habicht
CEO, Signifikans
How it Works
From first contact to signed consent—and every amendment after. eConsent makes the entire informed consent process easier for participants and study teams alike, with full integration into your study database.
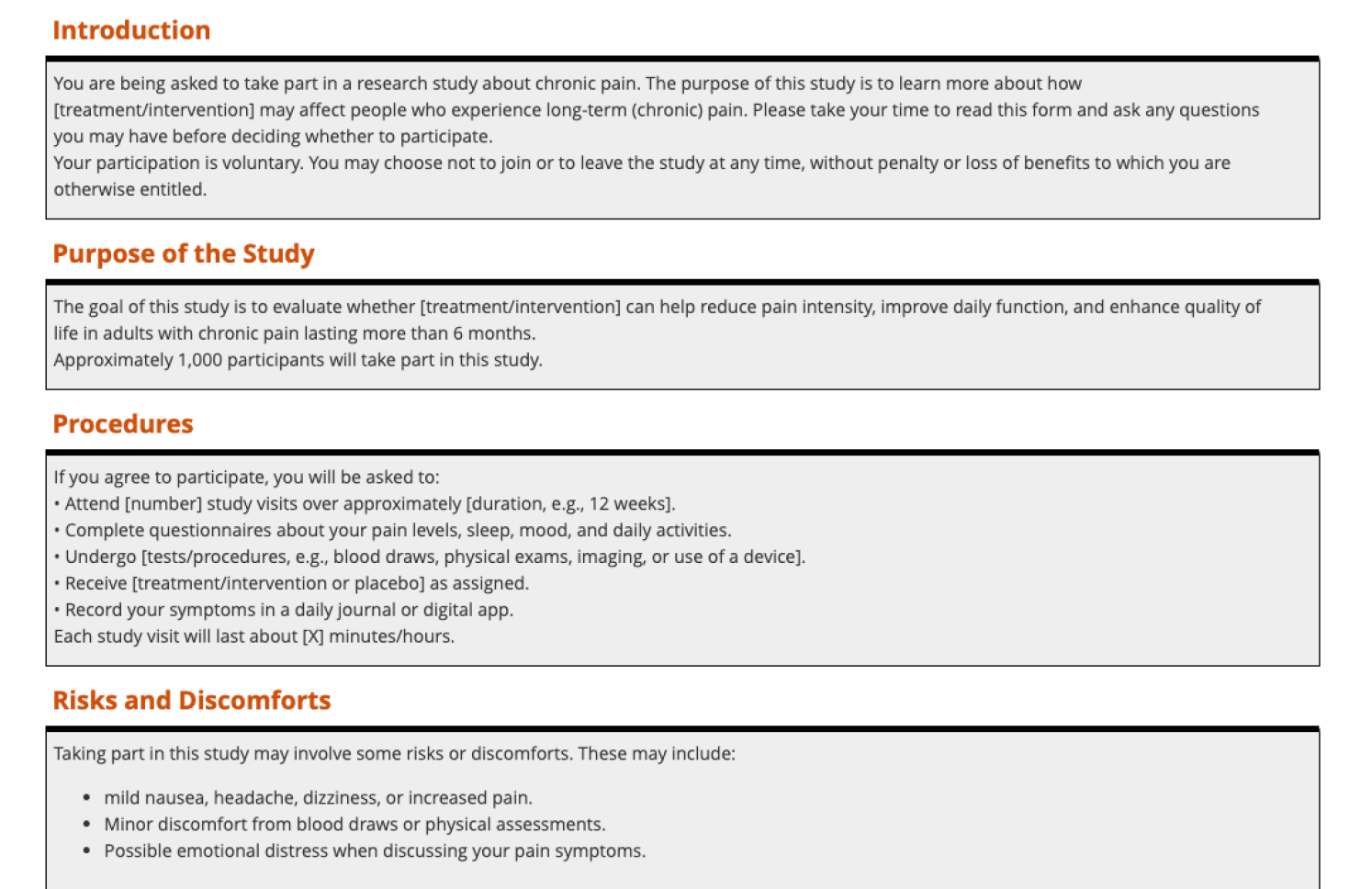
Create engaging consent forms
Build media-rich eICFs with images, audio, and video. Add comprehension checks to verify understanding before participants sign.
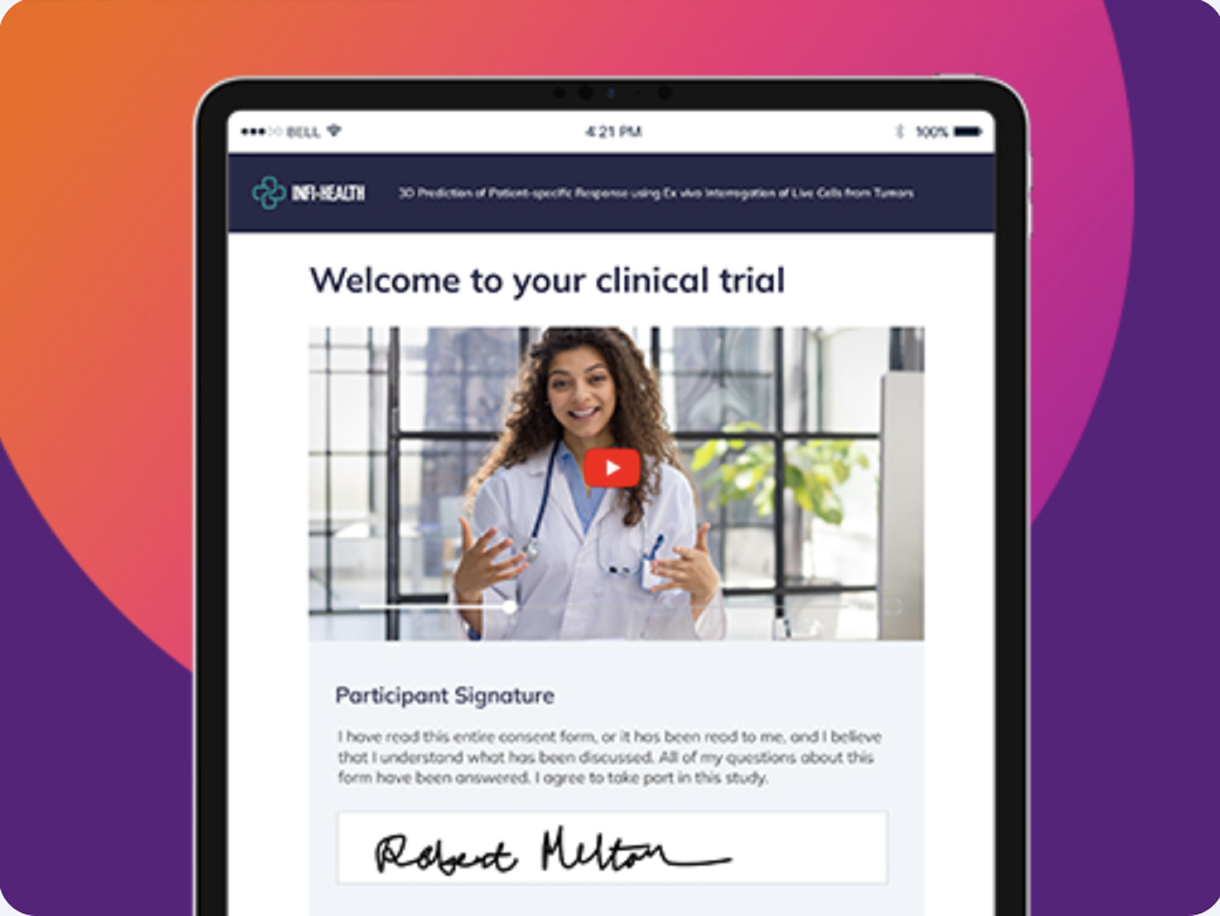
Deploy anywhere, anytime
Share a secure link. Participants review and sign on any device—in the clinic, at home, or on the go. No app to download.
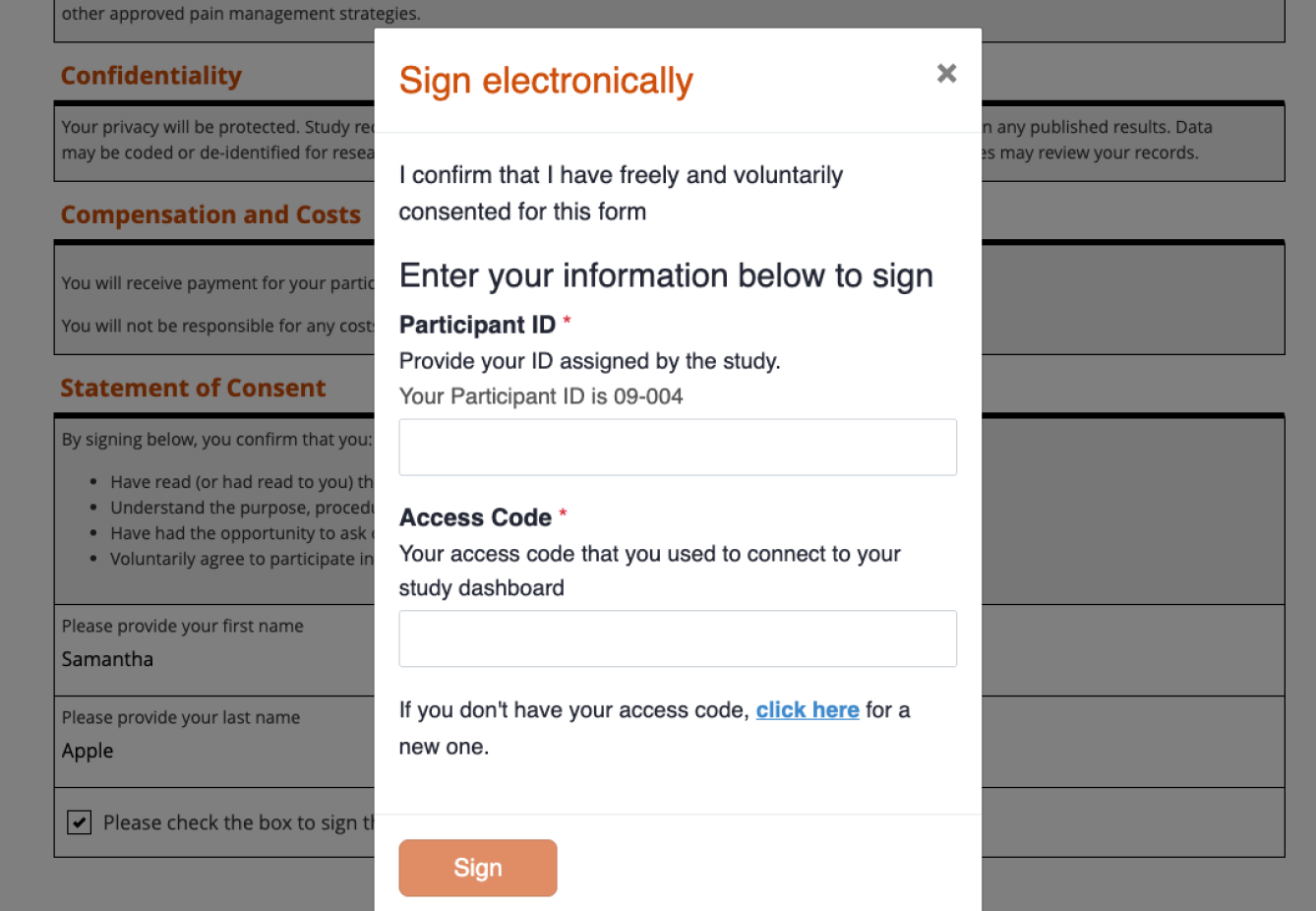
Track status in real-time
See exactly where each participant is in the consent process, right inside your study platform.
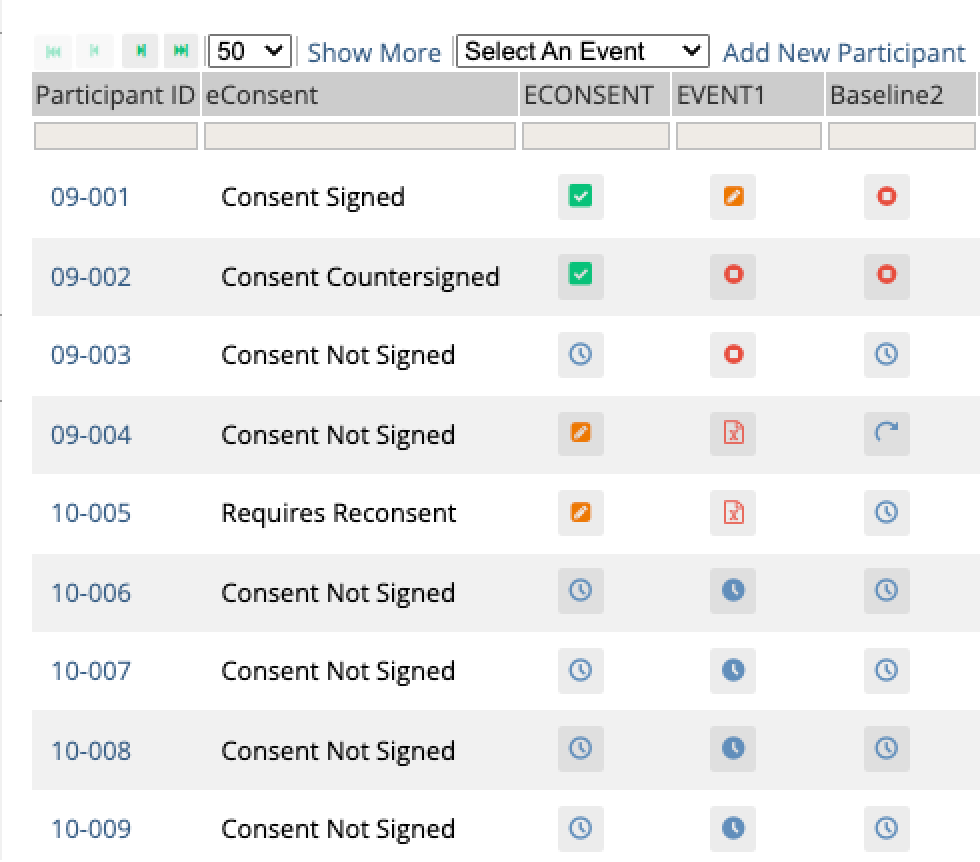
Stay compliant as protocols evolve
When amendments happen, automated re-consenting workflows trigger instantly. Participants receive signed copies automatically.
Create engaging consent forms
Build media-rich eICFs with images, audio, and video. Add comprehension checks to verify understanding before participants sign.

Create engaging consent forms
Deploy anywhere, anytime
Share a secure link. Participants review and sign on any device—in the clinic, at home, or on the go. No app to download.

Deploy anywhere, anytime
Track status in real-time
See exactly where each participant is in the consent process, right inside your study platform.

Track status in real-time
Stay compliant as protocols evolve
When amendments happen, automated re-consenting workflows trigger instantly. Participants receive signed copies automatically.

Stay compliant as protocols evolve
Ready to make informed consent truly informed?
Ideal For
- Decentralized and hybrid clinical trial
- Remote and in-person enrollment
- Global studies with multilingual populations
- Studies with frequent protocol amendments
- Trials requiring multiple signatures
- Teams needing improved participant comprehension
- Studies requiring HIPAA and GDPR compliance
Features
- Media-rich consent forms (images, audio, video)
- Embedded comprehension checks
- Works on any device—nothing to download or install
- Multilingual support
- Automated notifications and reminders
- Single or multiple e-signature capability
- Real-time consent status visibility
See more
- Automated re-consenting workflows for amendments
- Fully integrated with OpenClinica EDC
- Participants automatically receive signed digital copy
- Complete audit trail (21 CFR Part 11 compliant)
- HIPAA and GDPR compliant
- ISO 27001 and SOC 2 certified
- Transparent, modular pricing that fits your study volume
Resources:
Ready to make informed consent truly informed?
Share your consent workflow with us. We’ll discuss how eConsent creates better participant experiences while keeping your studies compliant, whether remote, in-person, or hybrid.