Randomization shouldn't slow you down
Complex randomization shouldn’t slow you down. Generate codes, manage lists, and allocate treatments directly inside your study—no separate systems, no manual tracking. With flexible methods and expert support to guide you through setup.
Who It’s For

Academic Researchers
Run complex adaptive designs without relying on separate statisticians or third-party vendors for every randomization list.

Sponsors & CROs
Manage multiple treatment arms, stratification, and re-randomization across multi-site studies—all within your EDC platform.

Sites & Coordinators
Randomize subjects in seconds with auto-generated codes that flow directly into your CRFs. No delays, no manual entry errors.
Benefits
Flexible randomization methods
Support simple, block, random permuted block, and minimization methods—whatever your protocol requires.
Stratify on anything
Stratify by age, sex, site, or any CRF variable. Unlimited treatment groups with custom allocation ratios.
Integrated with your study workflow
Randomize subjects directly within a CRF. Drug or randomization codes auto-generate into your study database.
Built for adaptive trials
Handle multiple randomizations, follow-up visit allocations, and evolving trial designs without rebuilding your system.
Blinded or unblinded
Run blinded studies with complete code list management, or operate unblinded when protocols require transparency.
Expert guidance when you need it
Complex randomization schemes? Our experienced statisticians guide you through setup, ensuring your design is configured correctly the first time.
Trusted by 1500+ sponsors, CROs, and research sites worldwide

"For ease of use, efficiency, and UI, OpenClinica is my first pick."
President

"What keeps us coming back is their commitment to giving us everything we need and nothing we don't, plus their unique user-friendly solutions including eCRF, ePRO and eConsent, making it easier for us and our clients to perform clinical trials."
Andreas Habicht
CEO of Signifikans
How it Works
From randomization list generation to code allocation at follow-up visits—all within your study platform. Configure your scheme, generate lists, and let sites randomize in real-time with complete audit trails and automated notifications.
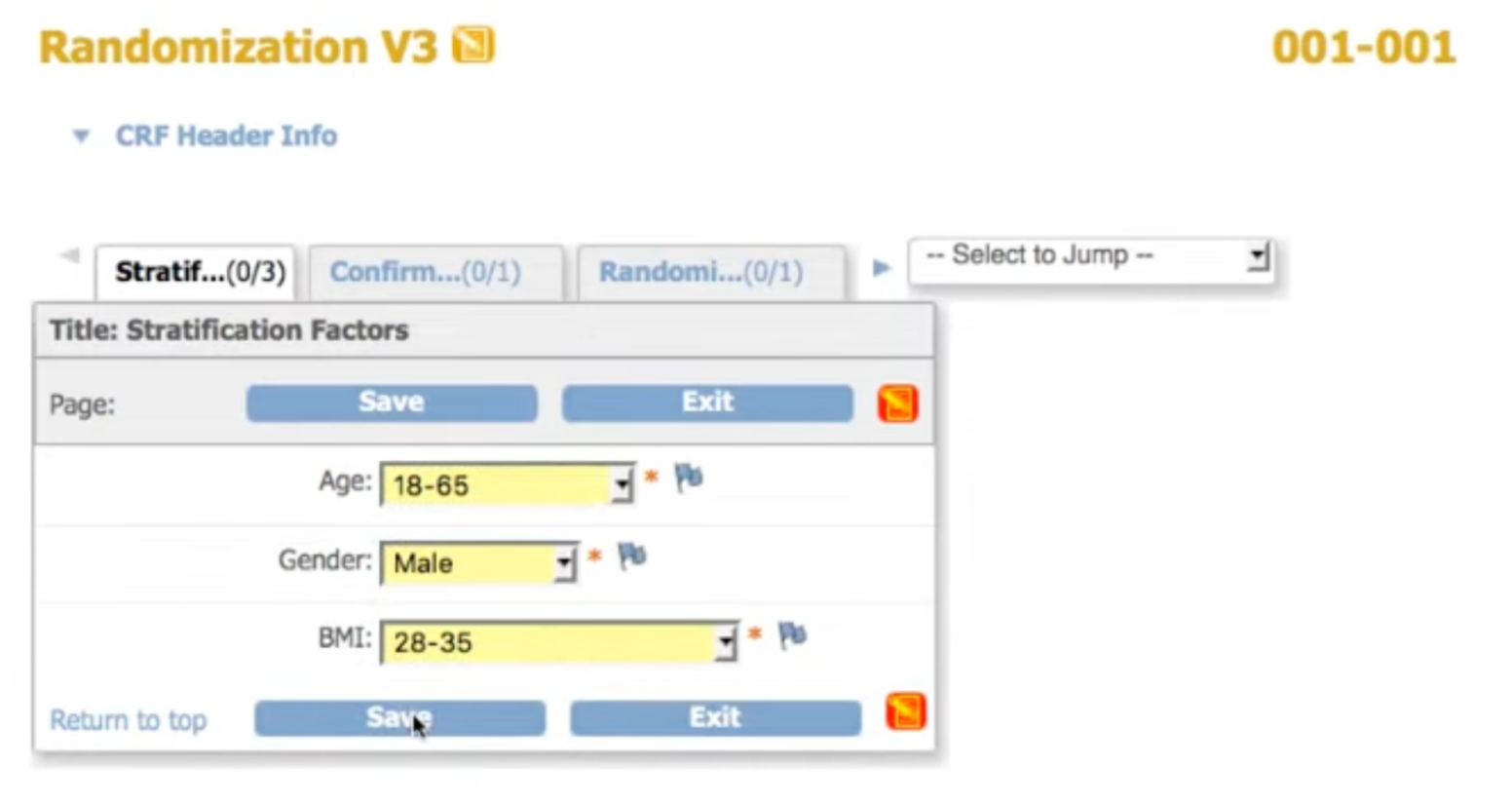
Configure your randomization scheme
Set your method, stratification variables, treatment groups, and allocation ratios. Our statisticians can guide you through setup.
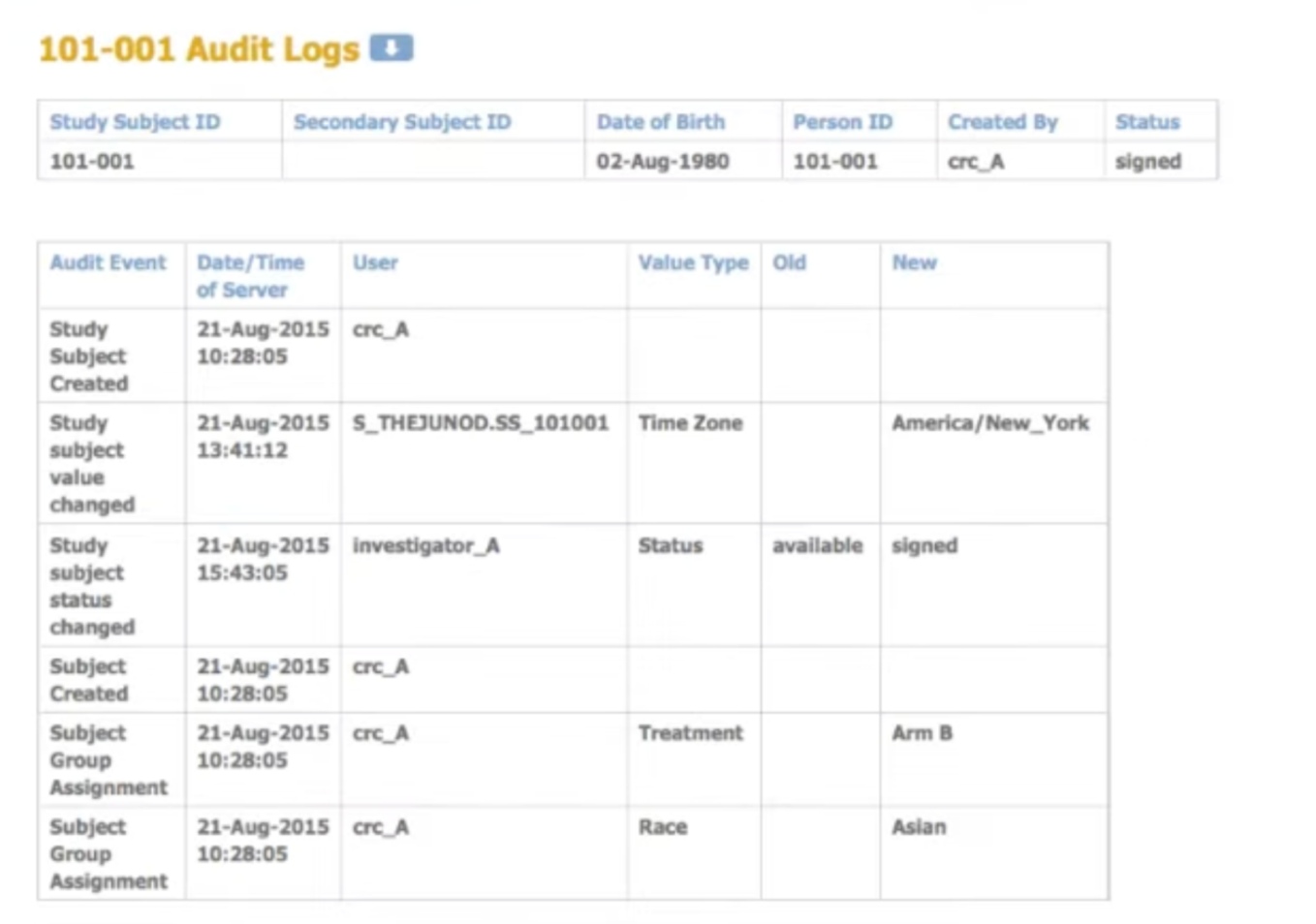
Generate and manage lists
Create randomization lists and code lists for labeling treatment kits. Manage codes centrally with full audit trails.
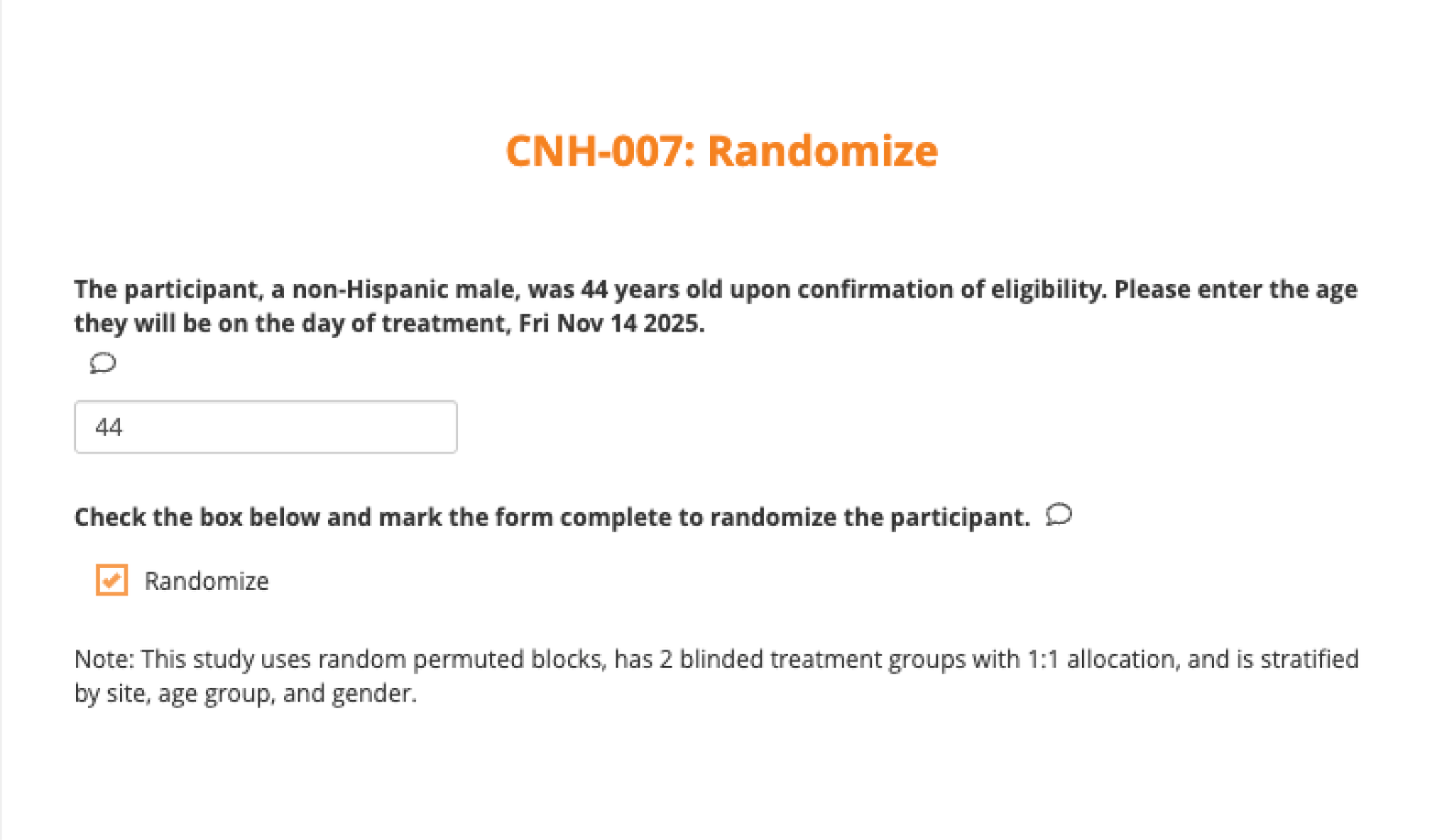
Randomize subjects in real-time
Sites randomize directly within a CRF. Drug or randomization codes auto-generate and populate into your study database instantly.
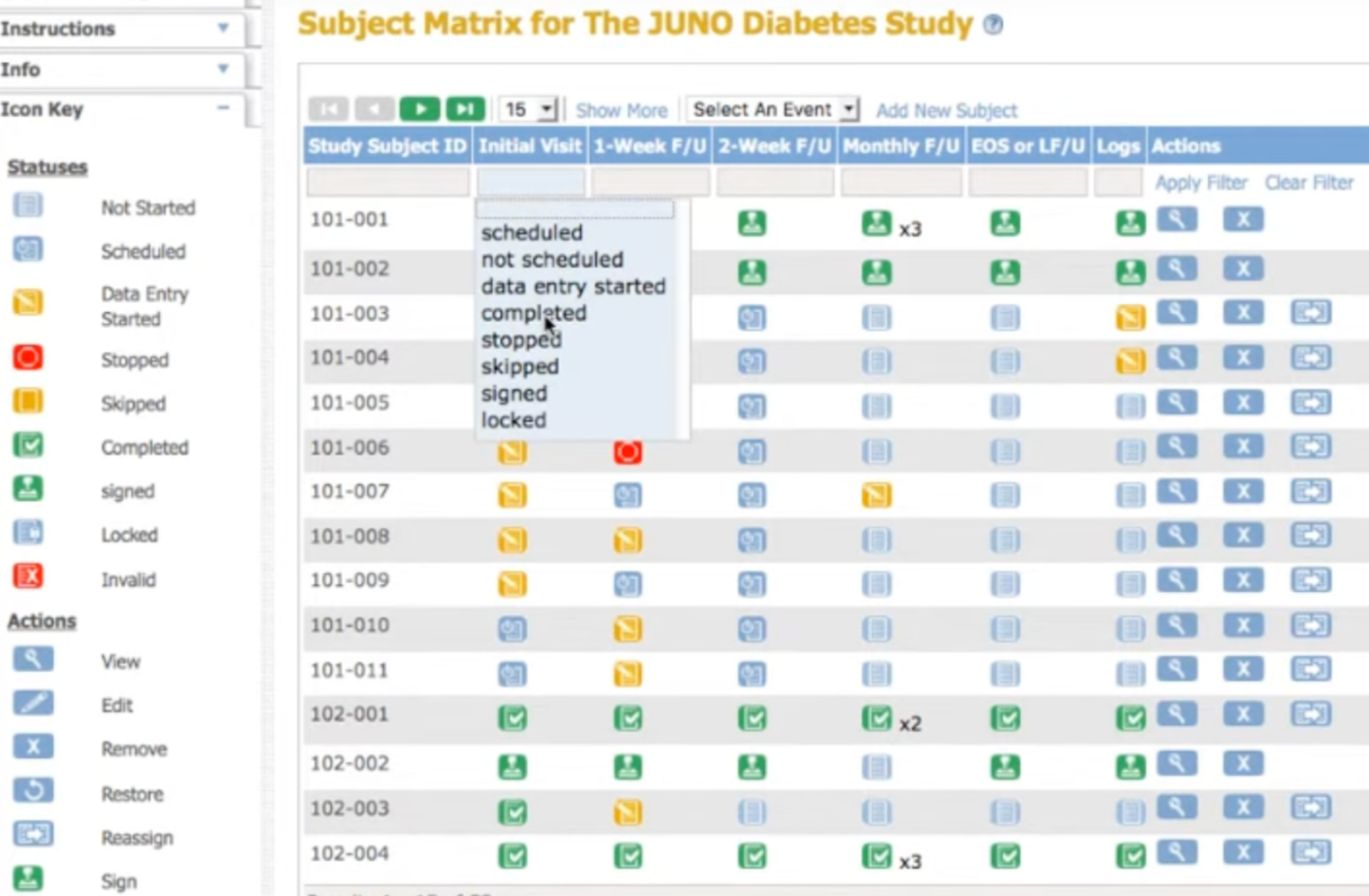
Track and notify automatically
See allocation status in real-time. Automated email notifications keep study teams and sites informed as randomization happens.
Configure your randomization scheme
Set your method, stratification variables, treatment groups, and allocation ratios. Our statisticians can guide you through setup.

Configure your randomization scheme
Generate and manage lists
Create randomization lists and code lists for labeling treatment kits. Manage codes centrally with full audit trails.

Generate and manage lists
Randomize subjects in real-time
Sites randomize directly within a CRF. Drug or randomization codes auto-generate and populate into your study database instantly.

Randomize subjects in real-time
Track and notify automatically
See allocation status in real-time. Automated email notifications keep study teams and sites informed as randomization happens.

Track and notify automatically
Ready to make randomization easy?
Ideal For
- Randomized controlled trials (RCTs)
- Adaptive trial designs
- Multi-arm and multi-stage studies
- Stratified randomization protocols
- Blinded and unblinded studies
- Studies requiring multiple randomizations
- Trials with follow-up visit allocations
- Teams managing their own randomization lists
Features
- Multiple randomization methods (simple, block, random permuted block, minimization)
- Stratification by any CRF variable (age, sex, site, custom fields)
- Unlimited treatment groups with custom allocation ratios
- Auto-generated randomization codes
- Code list management for treatment kit labeling
- Randomize directly within CRFs—codes auto-populate
See more
- Blinded and unblinded study support
- Follow-up visit code allocation
- Generated randomization lists
- Automated email notifications
- Real-time allocation tracking
- Complete audit trail (21 CFR Part 11 compliant)
- Fully integrated with OpenClinica EDC
- Expert statistician support for setup
- Transparent, modular pricing that fits your study volume
Hear from Our Expert
Ready to simplify randomization in your trials?
Tell us about your randomization requirements. Whether you need simple block designs or complex adaptive schemes, we’ll help you understand how our integrated approach keeps studies moving.