OpenClinica Share
Mobile sharing of medical records by participants for research
Mobile data sharing delivers access to a vast, diverse population & regulatory-grade, real-world data with less reliance on study sites. Powering large-scale, long-term, multi-system, real-world and complex clinical trials.
Benefits

Download
Participants download and add Share app to their smartphone, tablet and Apple Watch.

Connect
Participants use app to connect & authorize access to medical records.

Share
Participants tap to share specified records with study.
The future of research is individual participants
Extend your reach
Large-scale, long-term, real-world clinical trials are challenging — from recruiting enough people, to capturing enough data, to keeping everything on track over time. With Share, sponsors can tap into an ever-growing network of mobile-enabled institutions and interested potential participants.
- 850+ Global participating healthcare institutions
- 200,000+ healthcare locations
- Millions of people

Expand your research
- Aggregate health records for participants with complex histories, multiple providers and multiple health record systems.
- Auto-populate eCRFs with real-world source data in a single tap.
- Reduce site & monitoring burden.
- Finalize data faster.
- Accelerate study start.
- Maintain long-term momentum & engagement.
Data in the real world
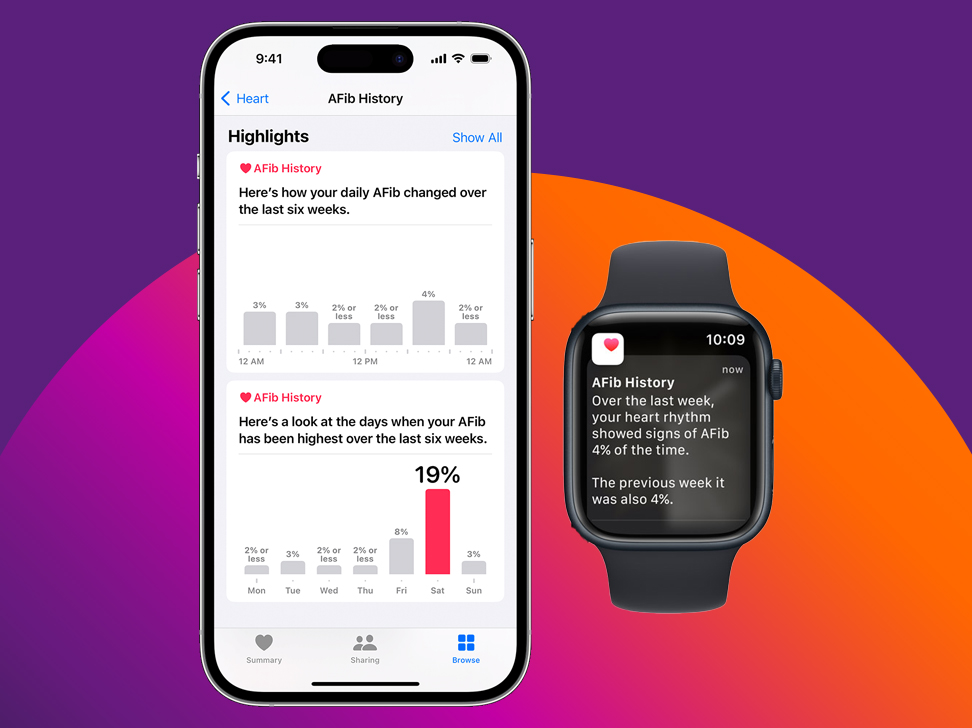
Amplify real-world data
In addition to sharing their medical records, participants can contribute real-world data from thousands of popular consumer apps, tracking everything from heart health and exercise to menstrual cycles and menopause.
Wearable devices like smartphones and watches also capture key environmental &. lifestyle factors — such as ambient sound levels, nutrition and sleep patterns — providing a holistic view of individual and population health.
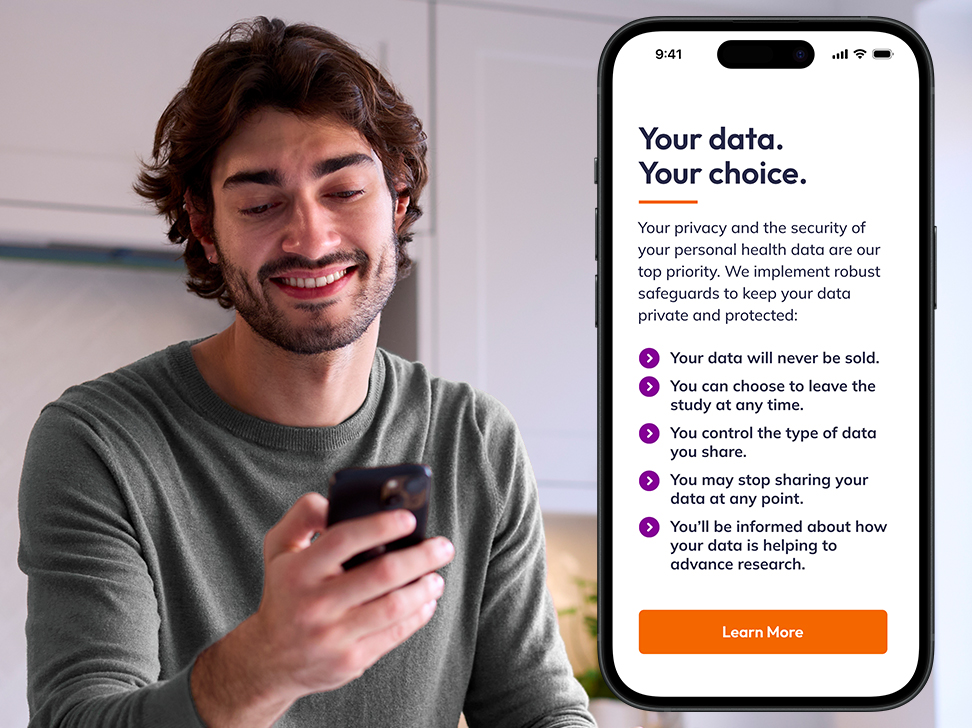
Protect data privacy
At the core of everything we do is a deep commitment to protecting health data. With Share, participants have full control over their data, with the ability to:
- Decide exactly what information they choose to share.
- Opt out at any time, instantly stopping data collection.
- Access clear, easy-to-understand privacy and security details, including what CRF 21 Part 11 compliance means for them and the study they’re part of.
The ultimate in patient-centricity

Better engagement
- Meet participants where they are.
- Engage in ways they want to be engaged.
- Lower the burden of participation.
- Empower participants to control their health data.
- Improve transparency & trust.

Better research
- Reach a broader audience.
- Engage more diverse & underrepresented populations
- Include participants from underserved and remote geographies.
- Improve accessibility by reducing barriers to participation.
- Foster greater inclusivity across various demographics & health conditions.
Simple. Secure. Compliant.
Lorem ipsum dolor sit amet, consectetur adipiscing elit. Ut elit tellus, luctus nec ullamcorper mattis, pulvinar dapibus leo.
Get Started with a Friendly Demo
Experience and explore our powerful AND easy platform at your own pace
Request a Demo
"*" indicates required fields