An audit trail in clinical research is a chronological record of all actions performed on clinical trial data, ensuring data integrity and regulatory compliance. The audit trail tracks all changes made to electronic data throughout its lifecycle, including who accessed the data, when they accessed it, and any actions performed. It’s a clinical trial technology solution that delivers an unalterable, transparent and traceable digital history of all data-related activities.
Clinical research audit trails aren’t exciting, but oh so important.
As clinical trials become more complex and adopt decentralized and hybrid approaches, the audit trail, in particular traceability, becomes more challenging and even more critical.
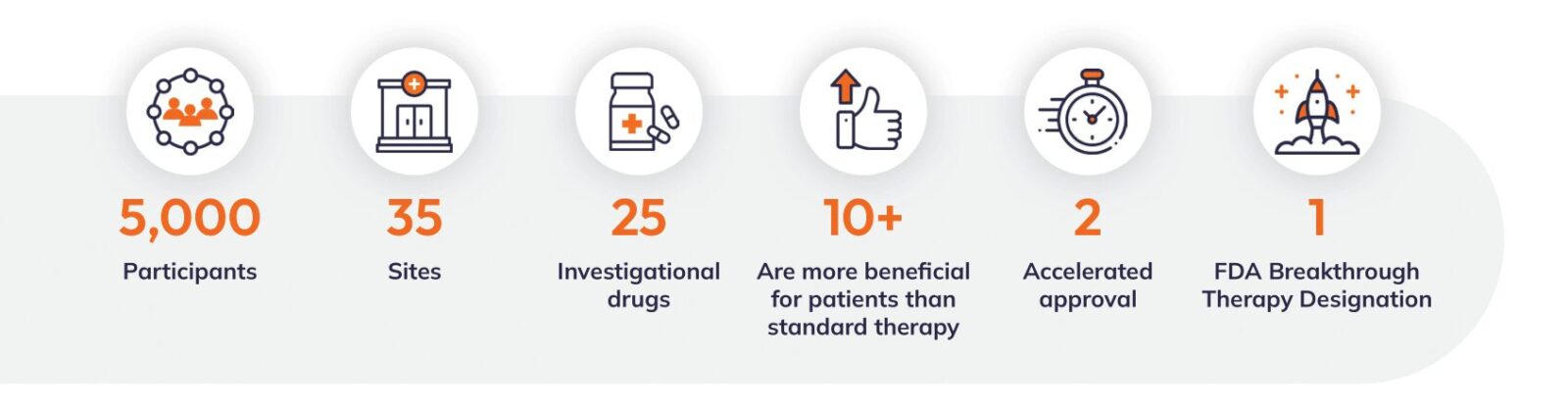
Take the I-SPY 2 clinical trial, for example. The study started in 2010 and by 2023, grew to 35 sites with 5,000 participants, successfully evaluating 25 agents, two of which gained accelerated approval and one granted breakthrough designation from the FDA. Study enrollment is still growing, with an anticipated study completion in 2031. Two decades of breast cancer clinical research means there could potentially be heaps of data changes.
Audit trails are one of the reasons why clinical trials like I-SPY 2 chose OpenClinica. Among other key attributes, we deliver clarity, compliance and oversight.
Understanding Audit Trails in Clinical Trials
Audit trails provide a record of all activities in a digital system. The U.S. Food and Drug Administration (FDA) describes an audit trail in their guidance on computerized systems used in clinical trials:
A secure, computer-generated, time-stamped electronic record that allows reconstruction of the course of events relating to the creation, modification, and deletion of an electronic record.”
As a part of the 21 CFR Part 11, the FDA has requirements for computer systems and traceability records. Likewise, the General Data Protection Regulation (GDPR) requires full traceability and the ability to create audit trails in the European Union (EU). Similarly, ICH E6(R2) impacts audit trails as it is an International Council for Harmonization guideline that provides a standard for the design, conduct, performance, monitoring, auditing, recording, analysis, and reporting of clinical trials.
In short, the four essential components of a compliant audit trail are who, what, when, and why. In more detail, an audit trail contains:
- Timestamps
- User identification
- Action description
- Reason for changes
- Link to records
- Original and new values
- System audit logs
- Data audit logs
- User audit logs
At the risk of understatement, audit trails mean much data needs to be tracked. In the case of the I-SPY 2 clinical trial, it’s more than 20 million data values and growing.
Source Tracking: The Foundation of Data Integrity
Source data in a clinical trial is the original records of clinical findings, observations and other trial activities. Without a doubt, it is critical to capture the origin of clinical data such as manual, device, ePRO and lab feed. It can be paper-based such as subject worksheets and physician notes or electronic. Examples are source tracking include monitoring the timeliness of data entry from source documents to electronic case report forms (eCRFs), tracking assessment completion times, and identifying unusual patterns in data entry. Precise source tracking delivers accountability, context and trust and ensures faster resolution of data queries and discrepancies.
Real-World Benefits of OpenClinica’s Approach
Audit trails ensure data integrity, regulatory compliance, transparency and accountability. They also can help detect errors, inconsistencies, or potential fraud in data. At OpenClinica, we know every change in your clinical data should be visible, secure, and auditable; audit trail transparency isn’t just a checkbox—it’s a core capability.
With site-agnostic audit tracking and centralized dashboards, OpenClinica helps sponsors maintain oversight in hybrid and decentralized trials. Regulatory audit trail requirements are not only met but exceeded through enhanced visibility, traceability, and real-time monitoring to ensure greater transparency and compliance.
Our approach has four key benefits:
- Improved sponsor confidence and reduced risk
- Faster issue resolution and query management
- True oversight in decentralized and hybrid trials
- Full transparency without overwhelming users
To learn more about why researchers choose OpenClinica’s clinical trial technology solutions, click here.



