Tools You Trust.
Support You Feel.
Even simple studies can turn into an IT nightmare fast—especially for small to midsize organizations. We built something different that works as hard as you do, with the flexibility and support to move with you every step of the way.
Real Talk from Real Researchers

"After 15 years as a customer, we can confidently say that OpenClinica continues to earn our trust. Their EDC has supported a range of projects for us, including pediatric studies, early-phase safety work, later-phase evaluations, and research aimed at enhancing gut health."
Andreas Habicht
CEO of Signifikans

"If not for OpenClinica and your team and your abilities, we would not be saving lives. We would not be able to reach the people who need it the most—the people who are literally hidden in the system and that are not seen."
Jordan Davis
Senior Policy Researcher, RAND Corporation
















Our Partnership, Our Promise
Clinical research software is only as good as the people behind it. Here’s what you get with OpenClinica, no matter which solutions you choose:
Your Customer Success Manager
Every client gets a Customer Success Manager who knows your studies, your team, and your goals. Not a rotating support queue—a real partner who's invested in your success.
Onboarding & Partnership
We don't just hand over a login. From study design to site training, we walk alongside you through setup and launch.
24/5 Application Support
Questions don't wait for business hours. Our support team is available 24/5 to help sites, coordinators, and data managers when they need it most.
On-Demand Training
Access our comprehensive Learning Management System whenever you need a refresher, want to train new team members, or explore new features.
We’re not just selling software. We’re building a partnership.
Modular Solutions Built for How You Actually Work
Set up studies in weeks, with flexible tools that think like you do. Choose what you need. Add more when you’re ready.
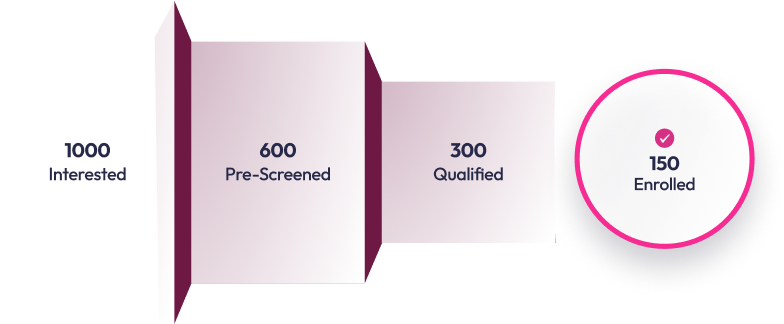
Reach enrollment goals without the guesswork
Predictable enrollment with precision-targeted outreach, smart pre-screening, and real-time analytics—at 3-5x lower cost per conversion than traditional methods.
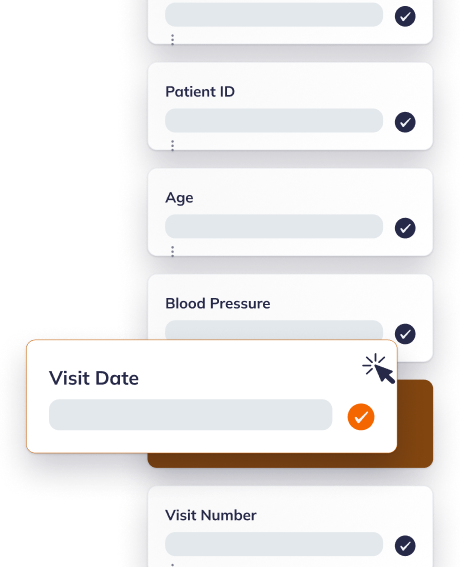
EDC that clicks immediately
Build studies in hours with drag-and-drop tools, templated CRFs, and real-time validation. Sites love the interface, and you'll love the 50% reduction in data queries.
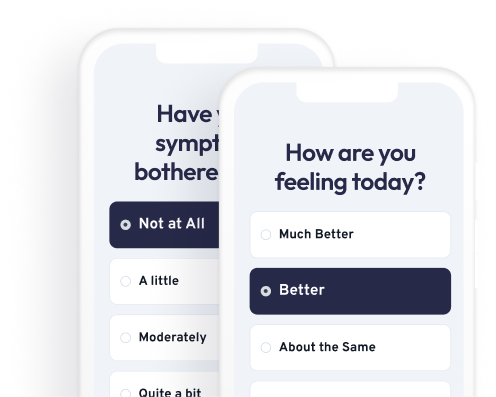
Patient-friendly reporting that teams trust
Mobile-friendly patient reporting with automated reminders and clean, flexible forms that increase compliance and reduce missing data.
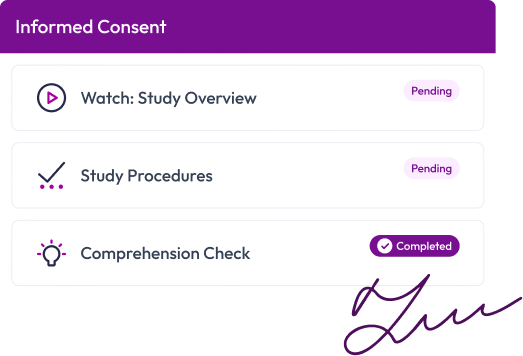
Consent participants with clarity and confidence
Multimedia-rich consent experiences with comprehension checks, automated notifications, and secure e-signatures—fully remote, in-person, or hybrid.
Real-time randomization that keeps studies moving
Simple to complex randomization schemes with blinded or unblinded access, inventory management, and automated notifications—all integrated with your study data.
Source data from your EHR without copy-paste
Pull lab results, medications, and vitals directly from EHRs into your EDC, saving coordinators hours and reducing transcription errors.
Know if your study's on track without digging through reports
Real-time dashboards for enrollment tracking, query management, and site performance. With customizable reports that give every stakeholder the visibility they need.
Reach enrollment goals without the guesswork
Predictable enrollment with precision-targeted outreach, smart pre-screening, and real-time analytics—at 3-5x lower cost per conversion than traditional methods.

Recruit
EDC that clicks immediately
Build studies in hours with drag-and-drop tools, templated CRFs, and real-time validation. Sites love the interface, and you'll love the 50% reduction in data queries.

EDC
Patient-friendly reporting that teams trust
Mobile-friendly patient reporting with automated reminders and clean, flexible forms that increase compliance and reduce missing data.

eCOA
Consent participants with clarity and confidence
Multimedia-rich consent experiences with comprehension checks, automated notifications, and secure e-signatures—fully remote, in-person, or hybrid.

eConsent
Real-time randomization that keeps studies moving
Simple to complex randomization schemes with blinded or unblinded access, inventory management, and automated notifications—all integrated with your study data.

Randomization
Source data from your EHR without copy-paste
Pull lab results, medications, and vitals directly from EHRs into your EDC, saving coordinators hours and reducing transcription errors.

EHR-to-EDC
Know if your study's on track without digging through reports
Real-time dashboards for enrollment tracking, query management, and site performance. With customizable reports that give every stakeholder the visibility they need.

Reporting & Analytics
Who We Work With
From university and hospital labs to sponsors and CROs

University researchers and academic medical centers running investigator-initiated studies. Flexible pricing that works with grant budgets.

Pharma, biotech, and medtech companies from pre-seed startups to established players. Scalable solutions that grow with your pipeline.

Contract research organizations managing multiple studies across diverse therapeutic areas. Multi-tenant capabilities with centralized oversight.
Your Study, Your Way
Whether you build it yourself or let us handle the details, you get the same support: your own CSM, on-demand training via LMS, and 24/5 application support—all included.
Build it Yourself
Perfect for teams who want control. Our modular approach means you only build what you need—with templates and tools that let you move fast without sacrificing quality or compliance.
- Intuitive drag-and-drop study builder
- Pre-built CDASH CRF templates
- On-demand training via LMS
- 24/5 application support
- One-click study publishing
Let Us Build It For You
Perfect for lean teams. Focus on your science while our professional services team handles the technical details—all with transparent, predictable pricing.
- Experienced study build consultants
- Full-service CRF design and setup
- Protocol review and recommendations
- Validation documentation included
- Post-launch support and optimization