About two million medical devices in 7,000+ categories are on the market. According to the Food and Drug Administration (FDA), a medical device is anything that treats a patient without medication, ranging from a simple thermometer to a pacemaker to a hip prosthesis.
Medical devices are categorized into different classes based on the level of risk they pose to patients and users.
- Class I Medical Devices are low-risk devices that are simpler in design and pose minimal potential harm to users. Examples include tongue depressors, bandages, and elastic gloves. Most Class I devices are exempt from premarket notification requirements, but they are still subject to general controls to ensure safety and effectiveness.
- Class II Medical Devices are of moderate risk and require greater regulatory controls compared to Class I devices. Examples include powered wheelchairs, infusion pumps and certain pregnancy test kits. Some regulatory authorities also have an intermediate category, such as Class IIa or Class IIb, to further differentiate devices based on their risk level. Most Class II devices require premarket notification. Many Class II medical device trials are short – as little as one day – and have a small study population.
- Class III Medical Devices pose the highest level of risk and are subject to the most stringent regulatory controls. Examples include implantable pacemakers, coronary stents and heart valves. Class III devices usually require premarket approval to demonstrate safety and effectiveness before they can be marketed.
The number of clinical trials for medical devices has trended upwards. In June 2022, for example, data from the GlobalData’s medical devices clinical trials database showed there were 300 new medical device trials across the globe, up from 7 percent from May 2022.

Source: https://www.medicaldevice-network.com/marketdata/new-medical-device-clinical-trials-june-2022/
In June 2022, the largest percentage of medical device clinical trials was healthcare IT (15 percent) followed by cardiovascular devices (14 percent) and neurology devices (9 percent).
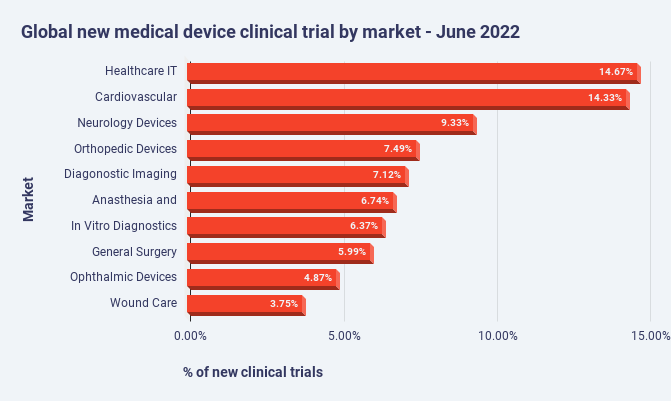
Source: https://www.medicaldevice-network.com/marketdata/new-medical-device-clinical-trials-june-2022/
How Medical Device Clinical Trials Differ from Drug Trials
Notwithstanding the fact that Class II and Class III medical devices go through a system similar to the drug clinical trials process, there are important differences such as drug clinical trials start with a Phase 1 trial for safety with healthy volunteers whereas medical device trials typically do not enroll healthy volunteers nor use a placebo for ethical and practical reasons.
Medical device trials have at least eight unique characteristics:
- Diverse Types of Devices: Medical devices encompass a wide range of products, from simple tools like scalpels to complex machinery like MRI machines. Each type of device may require different testing methodologies and considerations.
- Regulatory Framework: The regulatory pathway for medical devices, particularly in terms of approval and clearance, differs from pharmaceuticals. In the United States, for example, the FDA under the Center for Devices and Radiological Health (CDRH) regulates medical devices, and the approval process involves different criteria and procedures compared to drugs.
- Variability in Study Design: The design of clinical trials for medical devices can vary significantly based on factors such as device complexity, intended use and potential risks. Unlike drug trials, which typically follow a Phase I, II and III progression, device trials may have different phases or may not strictly adhere to this structure.
- Endpoint Measurement: Determining appropriate endpoints for medical device trials can be challenging. While efficacy and safety endpoints are common in pharmaceutical trials, medical device trials may also need to consider usability, functionality, durability and other device-specific parameters.
- Patient Population and Sample Size: Depending on the nature of the device and its intended use, recruiting suitable patient populations for medical device trials can be complex. Sample size calculations may need to account for factors such as device failure rates, user variability and the specific patient population being targeted.
- Blinding and Control Groups: Achieving blinding in medical device trials, particularly for invasive devices or those with obvious physical attributes, can be difficult. Control groups may also present challenges, especially if the standard of care involves different devices or techniques.
- Long-Term Follow-up: Many medical devices are intended for long-term use, which necessitates long-term follow-up in clinical trials to assess safety, efficacy and device performance over extended periods.
- Post-Market Surveillance: Unlike pharmaceuticals, which typically undergo extensive pre-market testing, medical devices often undergo further evaluation after they have been introduced to the market. Post-market surveillance is crucial for monitoring device performance, detecting adverse events and ensuring ongoing safety and efficacy.
OpenClinica Solutions Are Aligned to the Unique Needs of Medical Device Clinical Trials
Through our suite of solutions – including OpenClinica Unite™, our EHR eSource integration, OpenClinica Share™, which enables patient-level data capture with single-click mobile sharing of health record data, and OpenClinica Participate™, our ePRO, OpenClinica has supported more than 20,000 clinical trials across 100+ countries. As the result of our work with medical device clinical trials, OpenClinica has come to intimately understand the unique characteristics of medical device trials.
For example, our solutions easily support the differing endpoints of medical device trials. Likewise, medical device clinical trials can come in high volume with a need for quick set up. The ability of users to utilize our drag-and-drop interface to define study protocols and to leverage pre-existing forms are two of the primary ways OpenClinica enables high volumes and short turnaround times.
Overall, the unique characteristics of medical devices, coupled with regulatory requirements and the diversity of device types, necessitate tailored approaches to clinical trial design, execution, and evaluation. OpenClinica can help. To learn more, email us.