It’s widely known that oncology clinical trials are more complex and complicated than other trials. There are five reasons that help to explain why.
1. Recruitment and Enrollment Barriers
Even though the vast majority of Americans are inclined to participate in clinical trials, less than one in 20 adult cancer patients enroll in cancer clinical trials. As the image below shows, the process of identifying patients who qualify for a study is far from easy.
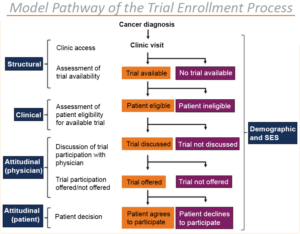
Image Source
The shift to precision medicine means many fewer patients will meet a study’s selection criteria. That’s why more sponsors are choosing to use digital and decentralized technology to create a bank of self-enrolled participants. This starts with a public URL and a basic screener study and ultimately can result in participants that are thoroughly screened, consented and ready to receive treatment.
2. Oncology Clinical Trial Design and Complexity
Clinical trials are generally increasing in complexity, but “oncology trials are outstripping the rest of the field due to enrollment challenges, protocol deviations and a burgeoning amount of data that are adding months to their timelines.”
According to the Tufts Center for the Study of Drug Development, the three phases of oncology trials are 14 to 18 months longer, on average, than other drug trials. That means nononcology trials typically run about eight years compared to nearly 12 years for oncology.
3. The Volume of Oncology Clinical Trial Data
Oncology trials generate much more data than other drug trials. For example, oncology trials have 3.1 million data points per protocol in phase 2 compared to 1.9 million in nononcology studies.
4. Adverse Event Measures
Oncology trials have different guidelines to classify adverse events. In nononcology studies, adverse events are categorized as mild, moderate or severe. For oncology trials, the National Cancer Institute’s Common Terminology for Adverse Events administers guidelines that classify adverse events from 1 to 5:
- Grade 1: Mild adverse events
- Grade 2: Moderate adverse events
- Grade 3: Severe adverse events
- Grade 4: Life-threatening or disabling adverse events
- Grade 5: Death-related adverse events
5. Efficacy Endpoints Differ
In nononcology trials, the common efficacy endpoint is the determination that a drug has effectively treated the condition. That’s partly true for oncology clinical trials. Many oncology clinical trials have an expanded endpoint – extending or improving patients’ quality of life. Some also have a goal to reduce the treatment side effects. Other oncology endpoints are: overall survival, progression-free survival and time to progression, time to progression, disease-free survival, event-free survival, time to treatment failure, time to next treatment, duration of clinical benefit, duration of response, objective response rate, quality of life, and more.
More than 50 percent of the cancer research centers certified by the National Cancer Institute choose to use OpenClinica solutions and services. To learn why, start your free demo today.

