Data Managers, do these statements feel familiar?
- “It’s 2017. I’m done with paper forms. My sites need fast, online data entry flows that match how they actually work.”
- “Protocols change. I need a quick, reliable way to make updates without having to worry about breaking things.”
- “I need to iterate on study and form revisions more quickly and collaboratively. I can’t afford to take my database offline for days, or even hours, just to add a field.”
- “I don’t want to test, revise, and re-test my forms only to have an error creep in when I go to publish. I need to build my form, test it from the site’s or participant’s view, and push it live in a straightforward, intuitive way. And duplicating it for others events and studies should take just seconds!”
- “I want my study team to easily see and take action on what needs to get done today. Then they can apply their brain power to the hard stuff.”
If so, you’re not alone. These pain points have added time and frustration to clinical research ever since we began putting information into databases. Most attempts to solve them have been inadequate. We’ve settled for making paper processes work “on a computer,” overlooking the drastic differences in how we think and behave–and what we’ve come to expect–on the web. In all likelihood, the past decade’s worth of web-based eClinical technologies have made your work faster, more complete, more efficient. But in moving away from paper, we’ve only traded crawling for walking (with ankle weights). It’s time to fly.
Today, we officially release the new OpenClinica, a phase shift in making research faster, more engaging, collaborative, and smarter. We’ve spent the last two years listening to users in various roles, from sponsor and data manager to study coordinator and participant. We’ve looked at eClinical through their eyes, testing beta versions of new features while refused to settle for any experience that wasn’t seamless, efficient – even beautiful. This, combined with a hell of a lot of hard work, have brought us to this day.
Whether you’re an existing OpenClinica user or working with another EDC solution, you can expect an experience that moves you from study idea to data extract in a logical and speedy way. The new OpenClinica retains and adds to the power of prior generations of OpenClinica, while entirely rethinking how you get work done.
From the beginning, you’ll be coached and guided through the intuitive new interface.

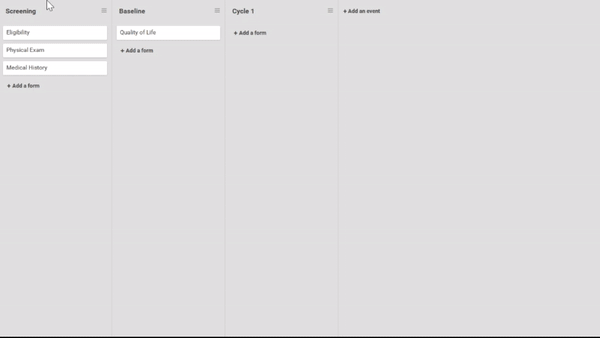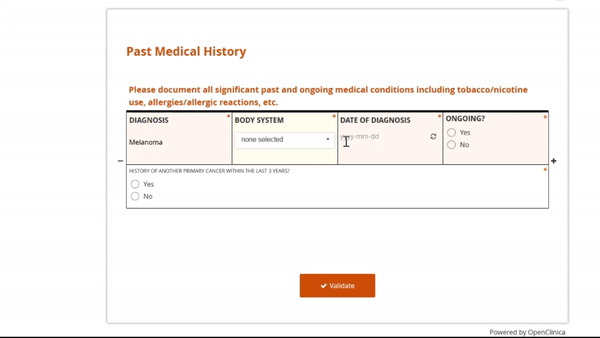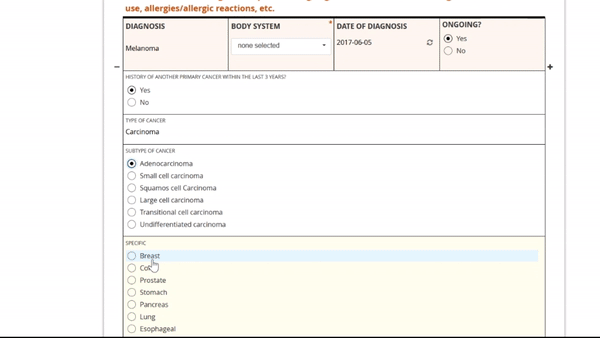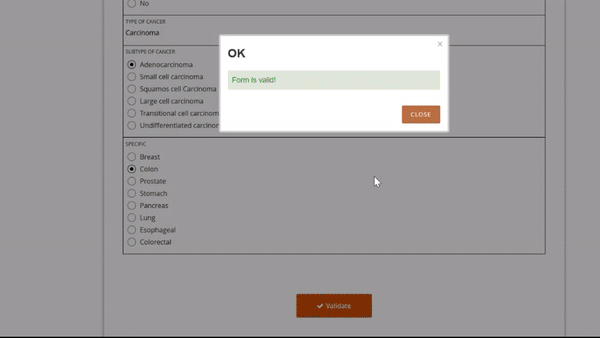
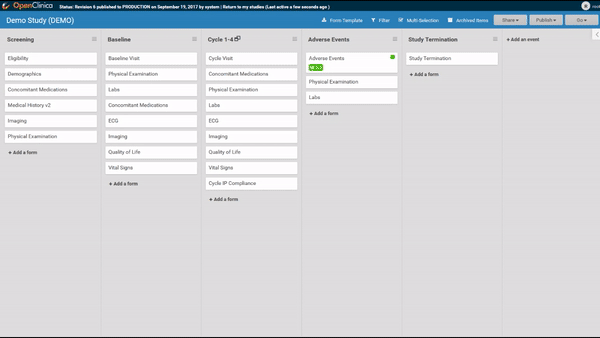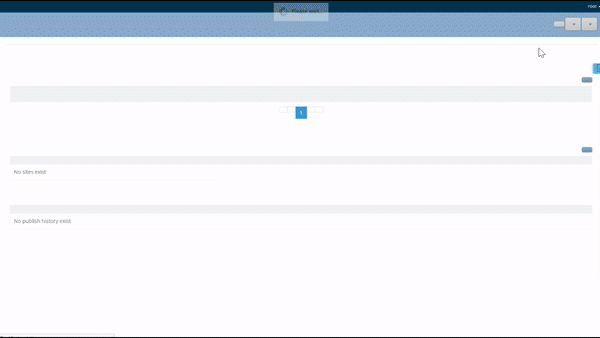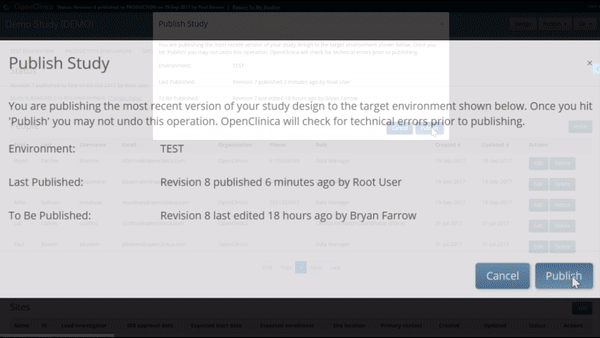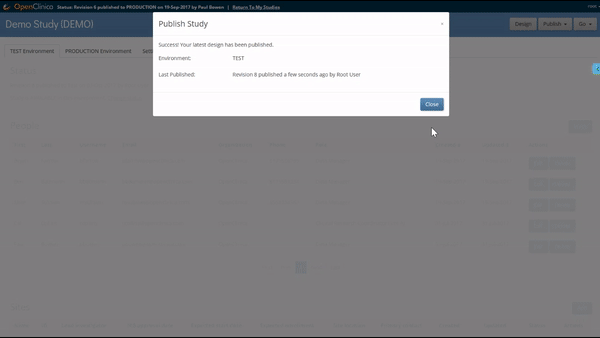
Start by ordering your study events.
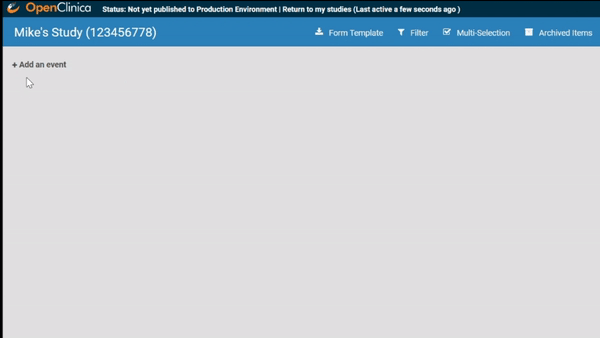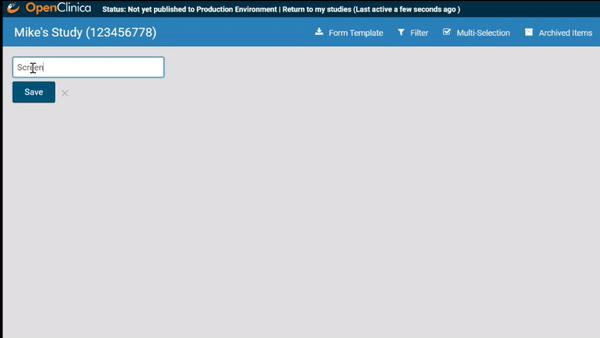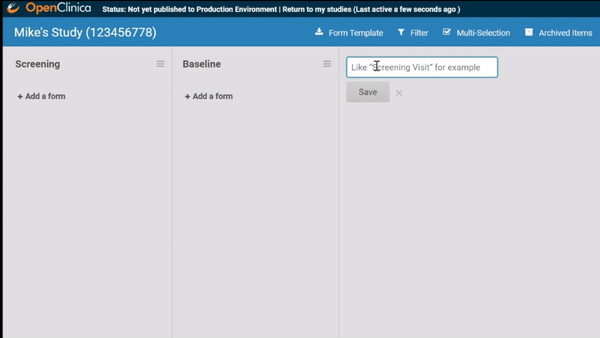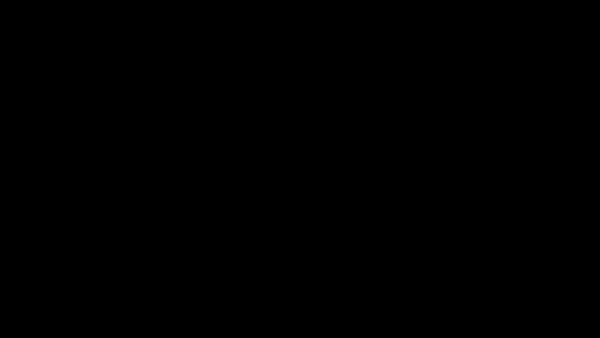
Associate forms with your events. Haven’t build your forms yet? No problem. Use a form card as a placeholder. The red exclamation point will remind you to upload a form definition before you publish.
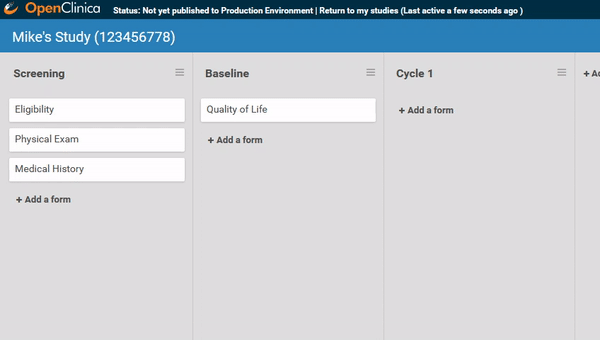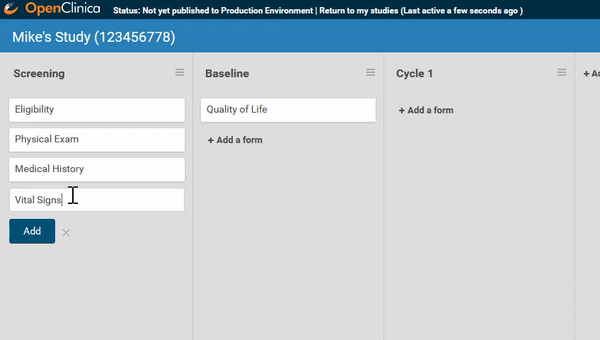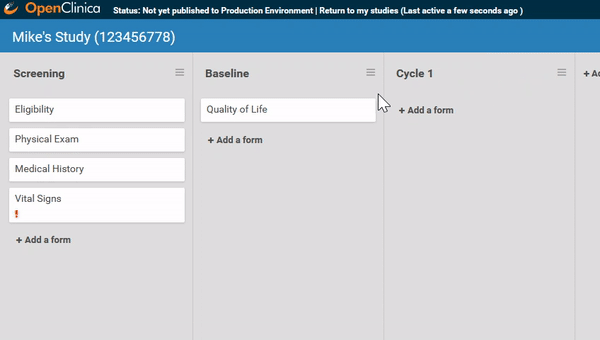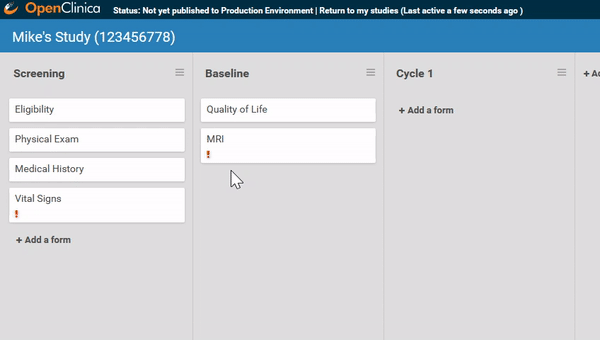
Need to move a form from one event to another? Remove a form? It’s as easy as dragging and clicking. (And nothing is ever “lost”, just archived, so you can restore what you need later.)
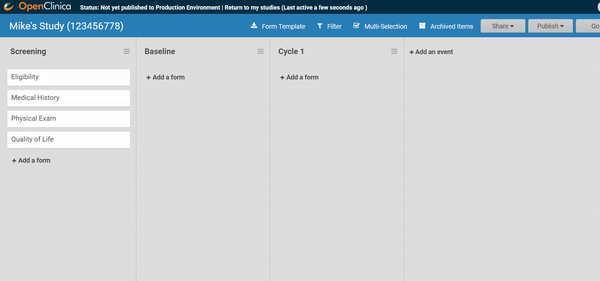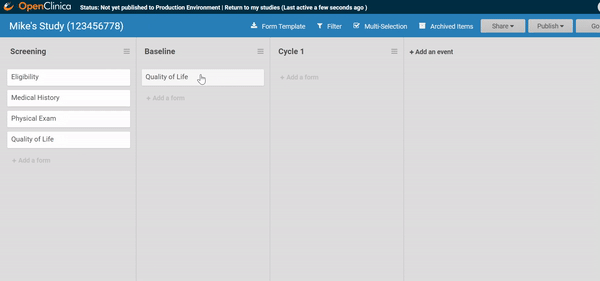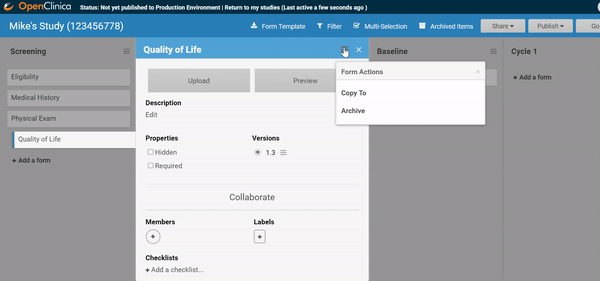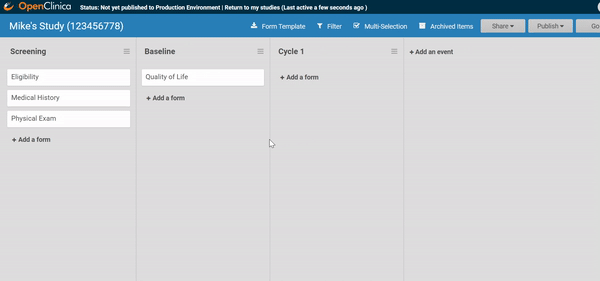
Adding and replacing a form definition is as easy as uploading. Once you’ve uploaded the form, preview it from the site’s or participant’s view, complete with edit checks and dynamic logic!
Ask for advice, or collaborate in real-time.
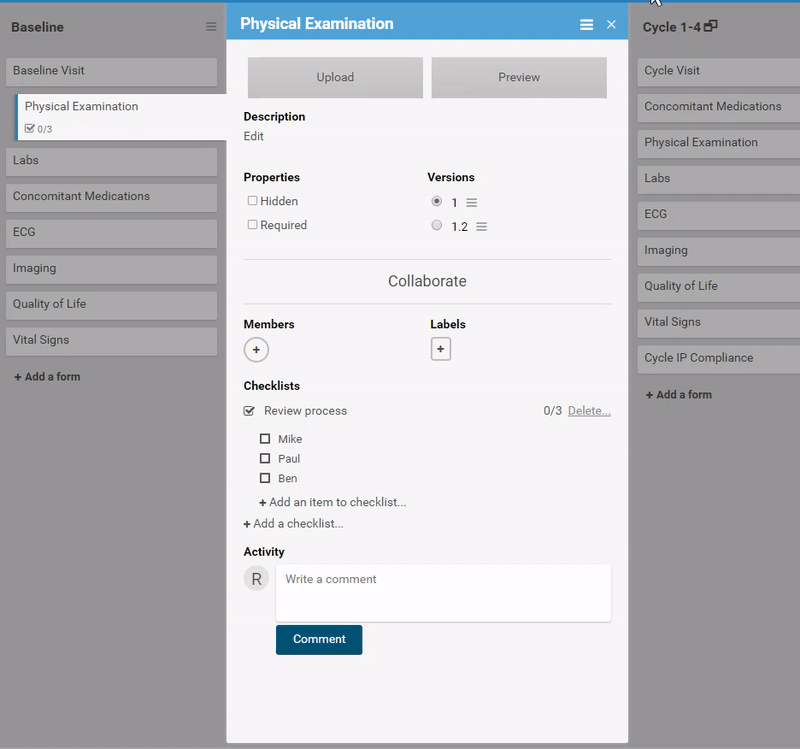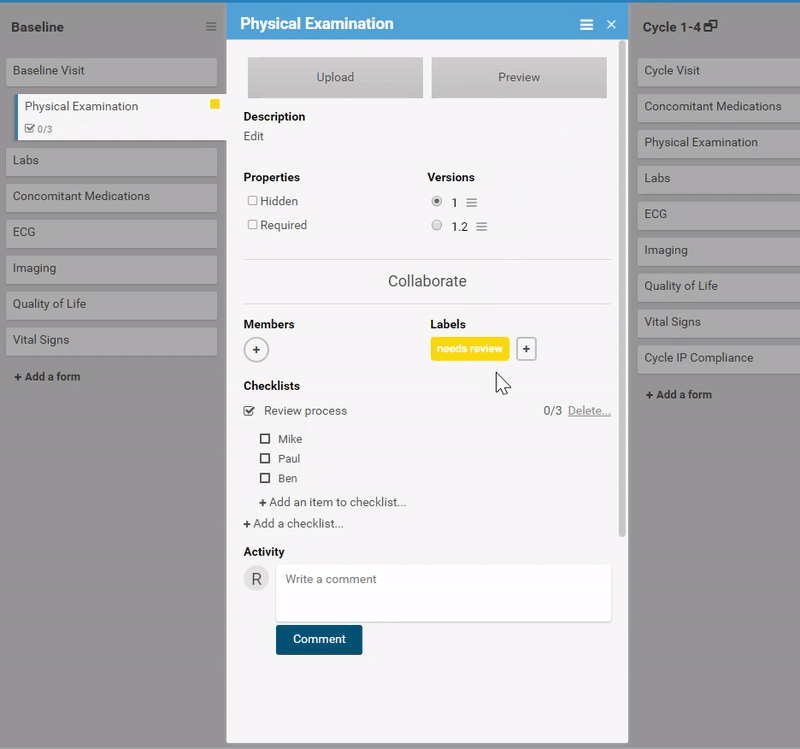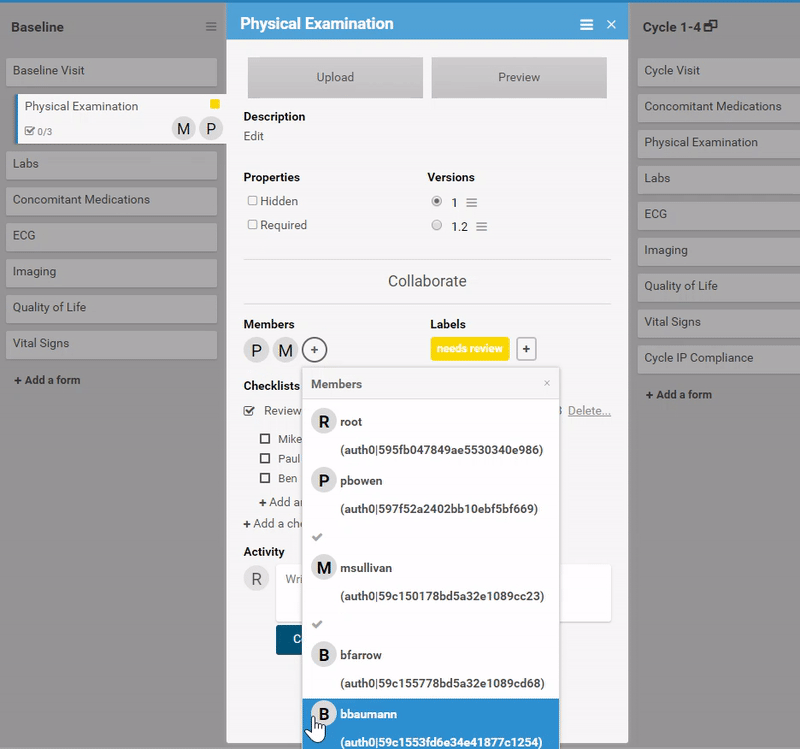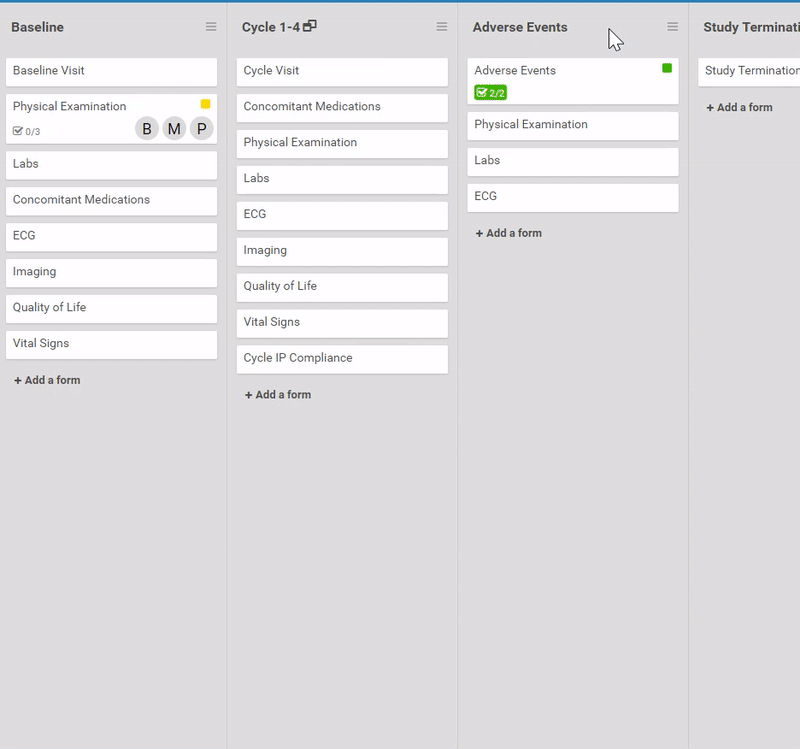
Like what you see? Publish it to test, then production. Same for changes and amendments that may come later.
And that’s just half the story. Once you’ve built your study, you’re ready to dazzle your sites and participants with the UI they’ve been waiting for. We’ll share that in our next post!
