- Solutions
- All Solutions
- Consent
- Electronic Data Capture (EDC)
- EHR-to-EDC Integration | eSource
- Electronic Patient Reported Outcomes
- Randomization for Clinical Trials
- Recruit | Patient Recruitment Solution
- Reporting
- Share | Mobile Data Sharing for Research
- Services: Study Configuration | Migration | Integration | Training
- Oncology Clinical Trials
- Resources
- Blog
- Events
- About
- Contact
Visualize and analyze all your EDC clinical data, from screening and enrollment to adverse event (AE) reporting and serious adverse event (SAE) alerts. Insight™ enables clinical data reports and dashboards that you can create yourself. You can even share reports with team members, sites, and sponsors.
-
 Track Enrollment in Real Time
Track Enrollment in Real TimeLet Insight™ automatically maintain your study enrollment reports.
-
 Receive Alerts of Adverse Events (AE)
Receive Alerts of Adverse Events (AE)Insight™ lets you and your safety officers know when adverse events are reported, day or night.
-
 Focus for Your Clinical Research Assistants (CRAs)
Focus for Your Clinical Research Assistants (CRAs)Use dashboards and alerts for more effective monitoring and source data verification.
-
 Easily Share Reports with Sites and Business Users
Easily Share Reports with Sites and Business UsersAutomatically send performance metrics and detailed clinical data reports on everything from open queries to safety alerts.
-
 Shred the Spreadsheet
Shred the SpreadsheetStop wrangling your data with slow, cumbersome spreadsheets. Reduce the churn and rework from static reports and listings leveraging self-service exploratory clinical data analysis.
-
 Access ALL Your Data
Access ALL Your DataReport on all your clinical data, ePRO data , query data, monitoring data, and even audit log data. Interactive visualizations with one click exports.
-
 Get Higher Quality Data, Faster
Get Higher Quality Data, FasterProvide views on all the data early and often to facilitate rapid clinical data cleaning and ensure data quality.
-
 Easily Answer Critical Clinical Research Questions
Easily Answer Critical Clinical Research QuestionsClinical data reports with meaningful insights. Track and evaluate biomarkers. Identify trends, patterns, outliers, errors.
-
 Interact with Your Data
Interact with Your DataInteractive visualizations that allow you to drill into your data, from exploring cohorts down to individual patients.
-
 Enhance Protocol Compliance
Enhance Protocol ComplianceExploration of the key aspects (sites, bottlenecks) of the protocol. Ensures protocol adherence by identifying drop-outs and violations.
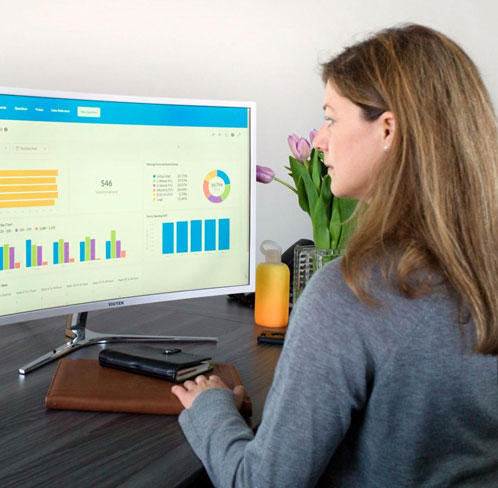
Along with training and support, OpenClinica Insight comes with customizable pre-created reports, including:
- Case Report Form (CRF) Completion by Event: Breaks down the number of forms completed vs. not completed by event and site.
- Missing Forms: List of required forms that have not been started and are in events that have been started.
- Query Aging by Month: Currently open queries created during that month, by site.
- Query Aging by Site: Open queries open, by the amount of time they’ve been open, by site.
- Query Aging by Study: Open queries categorized by age across the entire study.
- Source Data Verification (SDV) by Event: Shows forms requiring source data verification (SDV) by event.
- Source Data Verification (SDV) by Site: Shows forms requiring source data verification (SDV) by site.

The foundation of OpenClinica Insight is a well documented data warehouse that provides:
- Each OpenClinica study environment as a schema.
- Each case report form (CRF)/Item Group as a table, with items as columns.
- All non-removed data, plus audit logs for all data.
How does it work?
- Build point-and-click custom reports OR build them directly with SQL.
- Create unlimited clinical data reports and organize them into interactive dashboards.
- Allow access to stakeholders and sites based on fine-grained permissions.
- Automate alerts and updates. Email your reports to those who need them on a schedule you define or when a key event (e.g. serious adverse event (SAE) reported) occurs.

Here are a few questions you can answer using Insight™ business intelligence reports:
- Which “problem protocols” are failing to achieve enrollment targets?
- Which studies are currently in study start-up and how are they progressing towards activation?
- How are cohorts performing comparatively?
- How well is each site adhering to the protocol?
- Are there indications of possible safety signals I should be concerned about?
How will OpenClinica Insight reporting support my clinical data manager?
Data managers need quantitative snapshots of their study data. Without them, reporting on enrollment progress, planning for database locks, adverse event reporting, managing source data verification (SDV), and holding sites accountable to data entry and query resolution deadlines all become impossible.
Oftentimes, the burden of creating these snapshots falls on the resident Microsoft Excel expert or statistician, who will need days or weeks to create reports that end up being almost immediately out of date. Those days are over. With OpenClinica Insight, you can bypass this fiddling and proceed directly to immediate results you can use.












