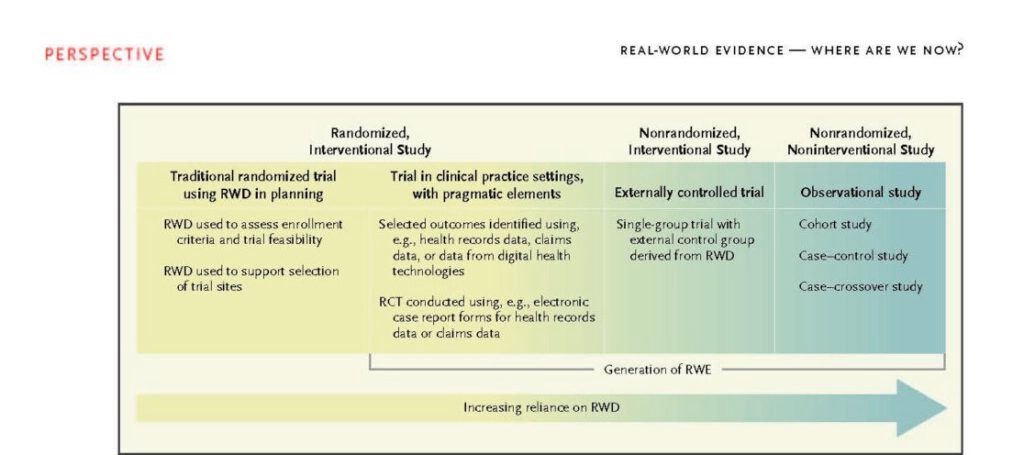
Notwithstanding ongoing confusion over the concepts of “real-world data” and “real-world evidence” in 2022, more than 5 years after the passage of the 21st Century Cures Act, the FDA continues to evaluate such data and evidence as it considers regulatory decisions. The reference to the OneSource Project on page two is the name of Quantum Leap Healthcare Collaborative’s program that is powered by OpenClinica Unite & EDC. Continue reading to learn more.
Download the PDF version here.
Article courtesy of The New England Journal of Medicine.