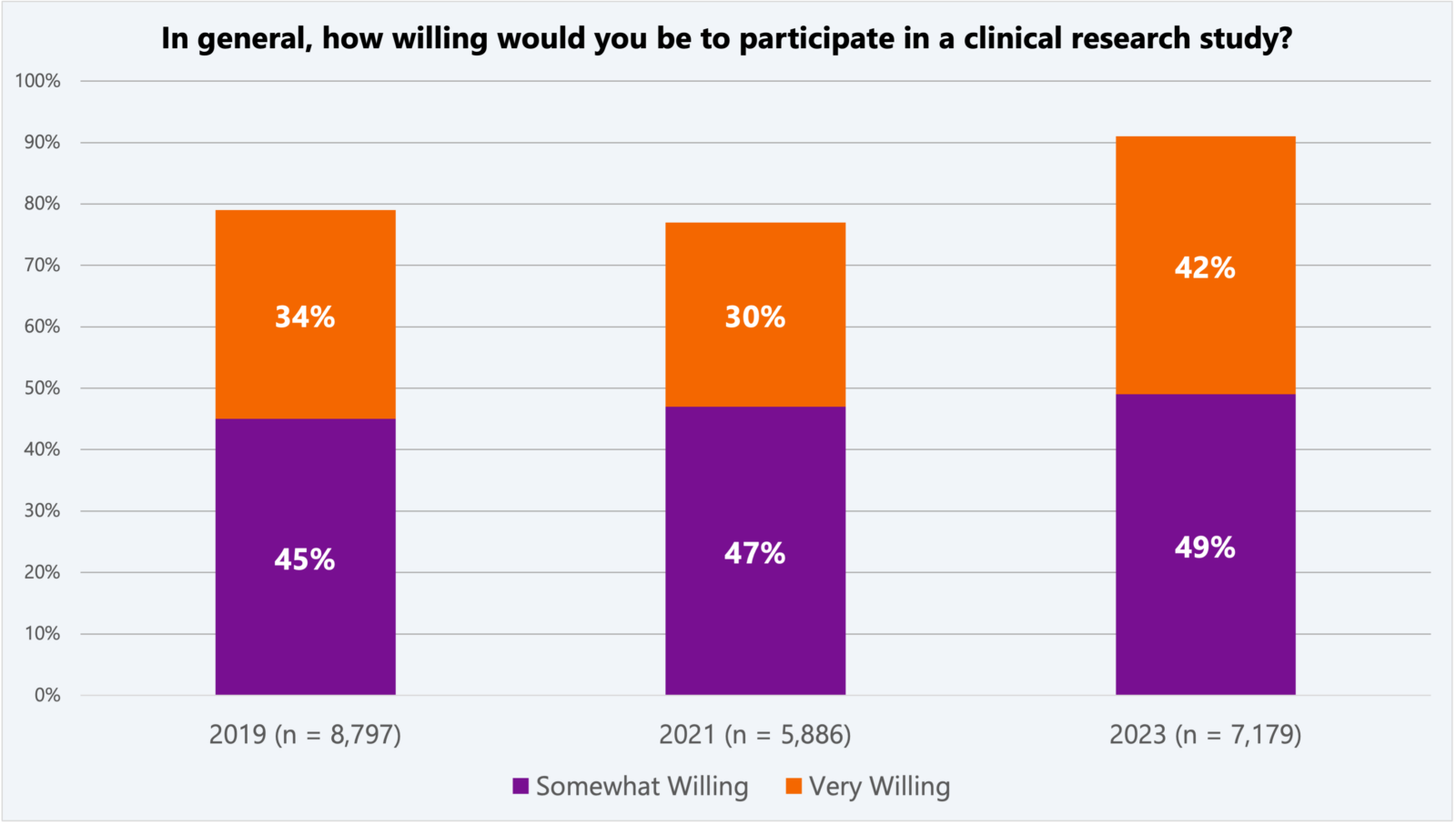
Interest in participating in a clinical research study is increasing, according to a global survey of 12,000+ conducted by the Center for Information and Study on Clinical Research Participation (CISCRP). Compared to 2021, the 2023 CISCRP study found that 42 percent of survey participants would be “very willing” and another 49 percent are “somewhat willing” to participate in a clinical trial.
https://www.ciscrp.org/services/research-services/perceptions-and-insights-study/
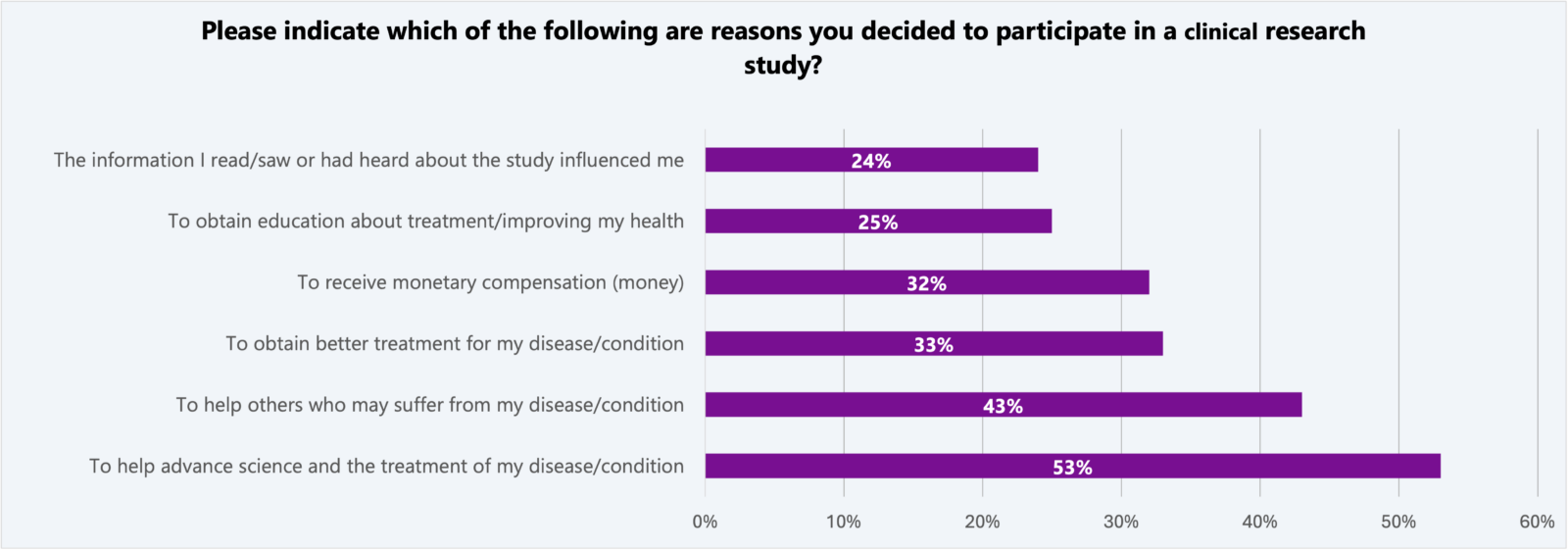
The same survey found the altruistic motivations remain the top reasons people chose to participate in a clinical research study.
https://www.ciscrp.org/services/research-services/perceptions-and-insights-study/
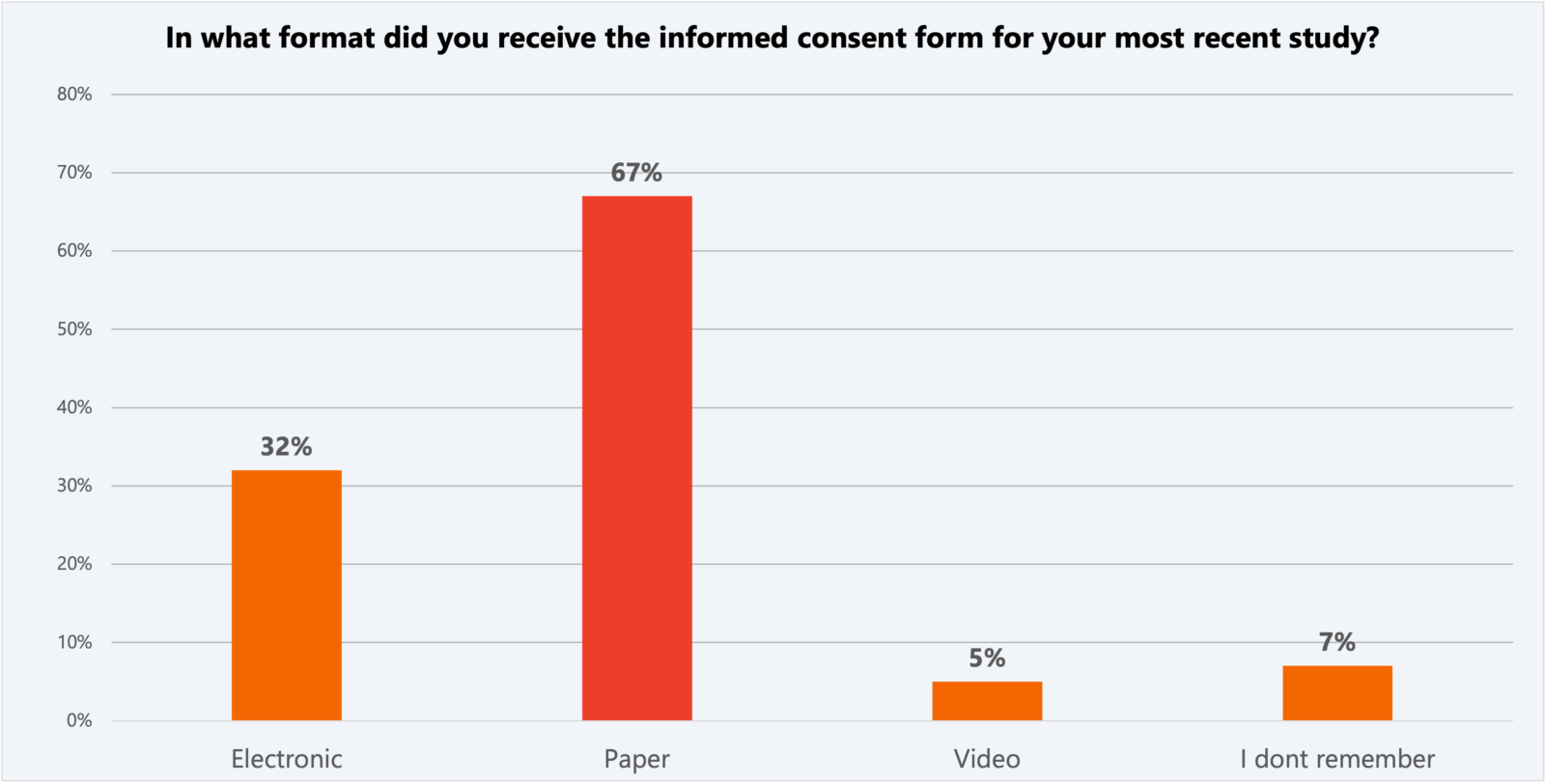
Interestingly, the 2023 CISCRP study revealed a decrease in the use of electronic and video consent formats compared to 2021. Two-thirds of respondents said they received paper informed consent forms.
https://www.ciscrp.org/services/research-services/perceptions-and-insights-study/
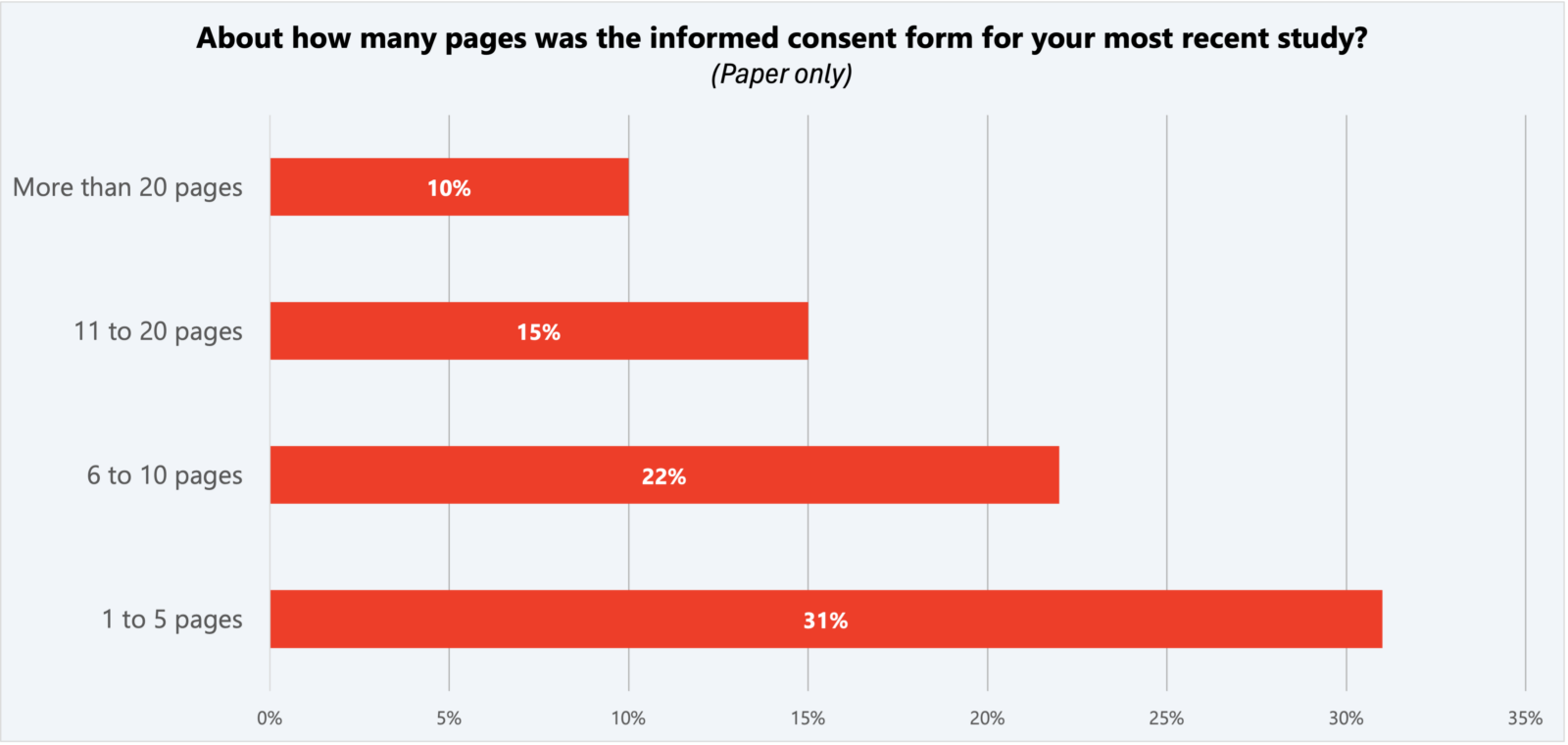
https://www.ciscrp.org/services/research-services/perceptions-and-insights-study/
At the same time, the study showed that the electronic consent form (eConsent) is perceived to be easier to understand than paper and video. Sixty-one percent of respondents said it was “very easy” and another thirty-one percent said it was “somewhat easy” to understand eConsent forms.

https://www.ciscrp.org/services/research-services/perceptions-and-insights-study/
Our work at OpenClinica with 15,000+ clinical trials and 3 million study participants has taught us that technology plays a distinct role in strengthening patient engagement in clinical trials. As discussed above, that starts with eConsent. Likewise, technology influences retention and engagement throughout the clinical trial.
How OpenClinica Uses Technology to Strengthen Patient Engagement in Clinical Trials
At OpenClinica, our solutions are designed to improve communication and convenience, and ultimately patient engagement.
- Electronic Consent (eConsent)is software that allows trial participants to read and sign informed consent documents online. Interactive, multimedia components can be used remotely or on site to empower patients to make informed consent and decisions, simplifying consent processes for participants and reducing the paperwork burden for clinical staff.
- Electronic Patient Reported Outcomes (ePRO) enables the receipt of data from trial subjects anytime, anywhere, on any device with no app to install and no need for a password, offering a frictionless way for patients to engage. With OpenClinica Participate™ ePRO, clinical trials can send friendly, just-in-time notifications and reminders through SMS or email, and patient-reported data that is fresh in a patient’s mind instantly becomes part of the overall study data set.
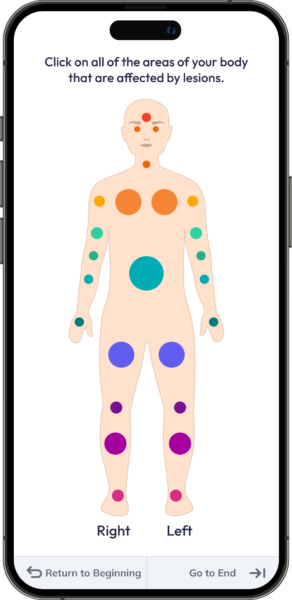
- Electronic Data Capture (EDC) is software used for collecting clinical trial data. As we showed in a recent demo, the patient-facing user experience is engaging and interactive. For example, clinical trial participants, as a part of a skin condition questionnaire, are asked to click on areas of the body that are affected by lesions.
Click on all of the areas of your body that are affected by lesions.
- Decentralized Clinical Trials (DCTs) use technology to communicate with study participants and collect data rather than relying on a site to carry out the study protocol day-to-day. Virtual technologies are needed to support DCTs such as remote leaning on the clinical trial protocol for practitioners and caregivers; digital transmission of data and collected outcomes; and virtualization of patient enrollment and other processes. DCTs rely on technologies such as eConsent, ePRO and EHR eSource integration.
We know DCTs help to alleviate the top study participation burden, which is travel.
When prompted on the most burdensome aspects of their participation, survey respondents ranked traveling to the study clinic as the top burden, with 3 out of 10 (29 percent) indicating that it was ‘somewhat’ or ‘very burdensome.”
To learn more about how OpenClinica strengthens patient engagement in clinical trials, visit: https://www.openclinica.com/blog/how-the-digital-front-door-benefits-clinical-trial-patients-clinicians-and-sponsors/