Let’s face the facts.
Patient enrollment in clinical trials is time-intensive and costly. In 2015-2016, the average cost to recruit one patient1 to a clinical study was $6,500+ and the average cost to recruit a new patient to replace a lost one was $19,000+. Today, patient recruitment accounts for 32 percent of costs making it the largest cost driver of clinical trials2.
In addition to being costly, patient enrollment also can be quite challenging. Approximately 80 percent of delays in clinical trial timelines3 are due to patient recruitment and retention. More than half of trials fail to meet their patient recruitment timeline. In the United Kingdom, for example, the National Health Services has “seen a 44 percent drop in participants recruited to commercial clinical trials in the past five years.”4
Cost drivers in clinical trials
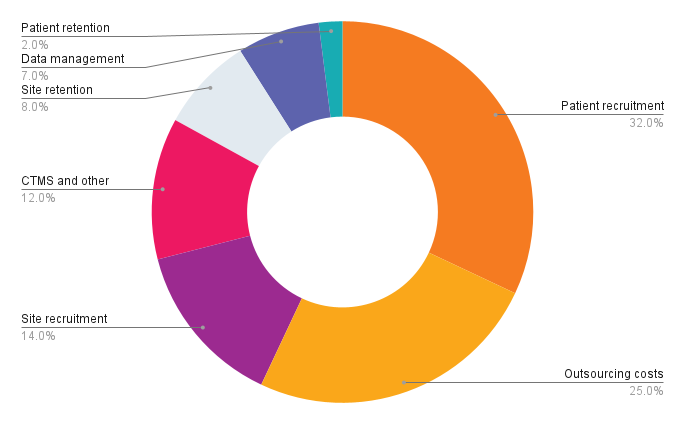
Source: Deloitte University EMEA CVBA. (2020). Deloitte Insights, Intelligent Clinical Trials.
Further, despite continued focus by sponsors and FDA guidance, clinical trial diversity is the lowest it’s been in ten years. Black/African Americans and Hispanic patients are “critically under-represented in trials using U.S. clinical sites.”5
Given the costs and complexity of patient enrollment, patient retention really matters – and it’s also quite challenging. We know 15 to 40 percent of patients6 enrolled in clinical trials drop out before the study is completed.
That’s why putting and keeping patients at the center of clinical trials is an increasingly important challenge to sponsors of clinical trials – even more so for those with therapies for chronic disease, patients with complex medical histories or trials for people with limited mobility. In other words, patient-centered clinical trials mean improving patient participation and satisfaction rates and designing trials to evaluate the things that actually matter to patients.
Why Patient-Centered Clinical Trials Matter
The evolution of consumerism makes patient centricity all the more important. Patients desire and expect the same level of personalization and ease with their medical care and clinical trials as they receive from their banks and streaming services. Given that the healthcare industry has historically lagged other industries, the use of consumerism techniques may pleasantly surprise your enrolled patients.
Better patient enrollment and retention leads to a more reliable trial, and that means sponsors are able to better predict and prove outcomes. At the same time, regulatory guidelines around diversity in trials are increasing. The 2023 omnibus spending bill requires diversity action plans to enroll more participants from underrepresented racial and ethnic populations into clinical trials. In other words, clinical trial enrollment needs to ”reflect the diversity of the population that is ultimately going to use the treatment.”7 As Bloomberg Law reported,
The idea is to encourage investigators to develop a strategy for reaching a broad study population on the front end8, instead of either failing to do so altogether, or acting later and increasing an already lengthy and costly clinical trial process.
As one reporter termed it, October 6, 2022 was “data liberation day”9 – the day they [patients] got their health data back.” Federal rules now require healthcare organizations to give patients “unfettered access to their full health records in digital format.” While this federal requirement may potentially seem like a burden to clinical trial sponsors, easier medical record sharing also leads to less redundant testing, a change that could reduce trial costs and patients’ burden of participation.
What’s more, the efforts to improve patient retention in clinical trials are adaptable to a variety of trial activities and types, including hypothesis generation, chart reviews, observational trials and the tracking of real-worth outcomes, especially over time.
In Practice: Patient-Centered Clinical Trials
So, what do patient-centered clinical trials mean in practice?
As most of us know all too well, almost nothing about interacting with the American medical system is easy. Sadly, that’s even more true in clinical trials. Today’s patients want digital tools that inform them and make their participation as seamless as possible.
Patient-centered clinical trials meet patients wherever they are – online, mobile and more – and engage patients in their preferred way, which for most, is digital first. This, in turn, helps to build patient trust. Other ways that clinical trials build trust are:
- Improving transparency around study design, aims and outcomes.
- Offering financial support, e.g., transportation assistance. In a September 2022 survey10 of nearly 2,000 patients and caregivers, 97 percent of patients surveyed said, in addition to general compensation for their participation, being reimbursed for meals and travel would appeal to them.
- Increasing patient engagement11. Listen and act on patient feedback when appropriate.
- Delegating data control to participants.
How to Make Clinical Trials More Patient-Centered
Start with informing patients of their rights to their data and then show them how to access the data and store it safely for themselves. It’s even more important for people with complex conditions and a complicated medical history. This commitment to a patient-centered approach improves the value of the trial to participants and helps to build trust.
Look to other industries and to your own consumer habits for clues as to how patients would prefer to interact with clinical trials. For most patients, that means mobile-first technologies for online screening, enrollment, consent, patient-reported outcomes and data-sharing of their medical records directly to the study database. These technologies have the added bonus of reducing the workload burden for clinicians and sites, freeing them to focus on patient care.
Without a doubt, technology and automation play a critical role in improving patient centricity in clinical trials.
To learn more about how OpenClinica can help make your clinical trials more patient-centered, click here.
Sources
- Moore, Zhang, Anderson, Alexander. (2018). Estimated Costs of Pivotal Trials for Novel Therapeutic Agents Approved by the US Food and Drug Administration, 2015-2016. JAMA. Retrieved from https://pubmed.ncbi.nlm.nih.gov/30264133/
- Deloitte University EMEA CVBA. (2020). Deloitte Insights, Intelligent Clinical Trials. Retrieved from https://www2.deloitte.com/content/dam/insights/us/articles/22934_intelligent-clinical-trials/DI_Intelligent-clinical-trials.pdf
- Desai, M. (2020) Recruitment and retention of participants in clinical studies: Critical issues and challenges
- Devlin, H. (2023). Patients losing out amid slump in NHS clinical trials, warn top clinicians. The Guardian. Retrieved from https://www.theguardian.com/science/2023/feb/27/patients-losing-out-amid-slump-in-nhs-clinical-trials-warn-top-clinicians
- IQVIA (2023). Global Trends in R&D 2023, Activity, Productivity, and Enablers. IQVIA Institute. Retrieved from https://www.iqvia.com/insights/the-iqvia-institute/reports/global-trends-in-r-and-d-2023
- Rites, J. (2020). Use virtual visits to enhance the patient centricity of clinical trials. MedCity News. Retrieved from https://medcitynews.com/2020/10/use-virtual-visits-to-enhance-the-patient-centricity-of-clinical-trials/
- FDA (2022). FDA Takes Important Steps to Increase Racial and Ethnic Diversity in Clinical Trials. FDA. Retrieved from https://www.fda.gov/news-events/press-announcements/fda-takes-important-steps-increase-racial-and-ethnic-diversity-clinical-trials
- Baumann, J. (2023). Diversity in Clinical Trials at FDA Gets a Boost From New Law. Bloomberg Law. Retrieved from https://news.bloomberglaw.com/pharma-and-life-sciences/diversity-in-clinical-trials-at-fda-gets-a-boost-from-new-law
- Casey, R. (2022). Call it data liberation day: Patients can now access all their health records digitally. STAT. Retrieved from https://www.statnews.com/2022/10/06/health-data-information-blocking-records/
- Elliott, L. (2022). Boosting Clinical Trial Appeal in Patient Communities Part 1: Rare Patient Voice FLASH Webinar Overview. CiSRP. Retrieved from https://www.ciscrp.org/boosting-clinical-trial-appeal-in-patient-communities-part-1-overview/
- Baumann, B. (2023). Patient Engagement: Opening the Digital Front Door to Clinical Trials. OpenClinica. Retrieved from https://www.openclinica.com/blog/patient-engagement-opening-the-digital-front-door-to-clinical-trials/

