Building Successful Studies: A Proven Framework
Last week, OpenClinica published a blog by our co-founder and chief operating officer, Ben Baumann, Meet OpenClinica’s Solutions Consultants. ICYMI,...
The anatomy of an electronic case report form (eCRF)
An electronic case report form (eCRF) captures clinical trial data. What are the key parts of an eCRF and what...
Empowering Clinical Trial Sites to Deliver Better Clinical Data, Faster
SUMMARY: In this free webinar, learn how to empower site staff by providing technology that automates your clinical trials –...
Challenges in Clinical Research Make the Case for Decentralized Clinical Trials (DCTs)
The State of Decentralized Clinical Trials (DCTs) A look at the challenges facing clinical trial sponsors and sites Decentralized clinical...
Master form design with these two on-demand webinars
Equipped with the right system, data managers today have more tools than ever before to capture high-quality right at its...
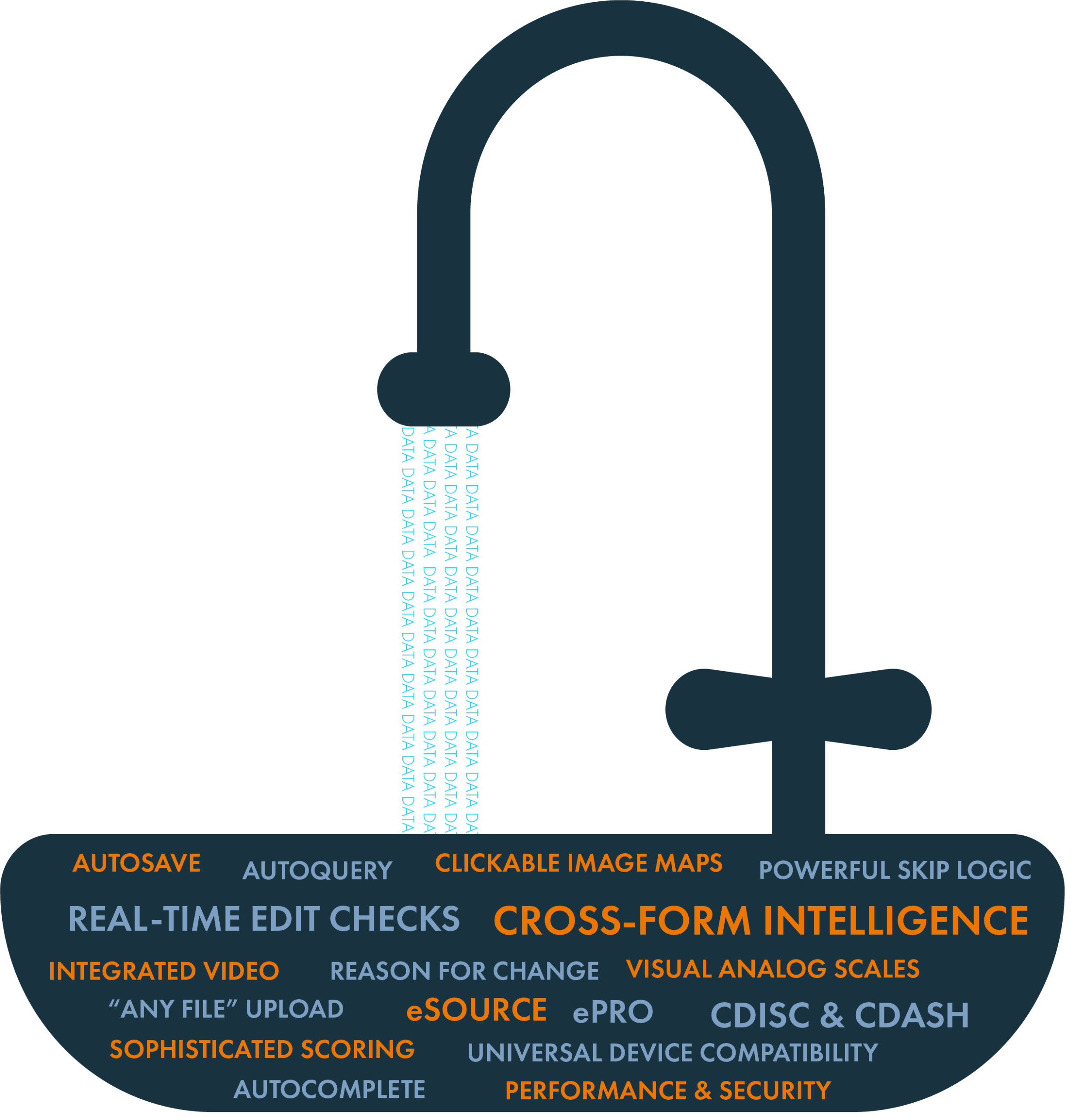
The Kitchen Sink: See everything OpenClinica forms can do for you
In just thirty minutes, explore all of OpenClinica’s form capabilities, each doing its part to ensure better data, faster! See...
Register for “Good Form: Designing Your eCRFs for Better Data, Faster”
Want to maximize data quality and speed? Focus on your forms. From layout to item order, skip logic to edit...
14 Best Practices for ePRO Form Design
Is there any term in data collection more despairing than “abandonment rate”? That’s the percentage of respondents who start inputting...