Efficiency & Quality: The Impact of CDASH eCRFS & OpenClinica EDC on Clinical Trials
It’s my privilege to share with you the article, Dissemination of CDASH eCRFs via the CDISC Electronic Case Report Form...
Four Ways to Strengthen Patient Engagement in Clinical Trials
Interest in participating in a clinical research study is increasing, according to a global survey of 12,000+ conducted by the...
Decentralized Clinical Trials: Finding the Best Technology Partner
Broadly speaking, there are three types of decentralized trials, and having the right technology partner can help with all of...
The Workhorse of Today’s Clinical Trials
The electronic data capture platform (EDC) is undoubtedly the workhorse of decentralized clinical trials. That’s why the EDC figures prominently...
Letter from our CEO
Recently, a few of us at OpenClinica received an email from a long-time customer who was leaving her organization for...
Reporting on your Data in Decentralized Clinical Trials (DCTs): A Checklist
A checklist for ensuring your decentralized clinical trial can report on and analyze its clinical data The following checklist offers...
OpenClinica at ACRP 2022: What to expect
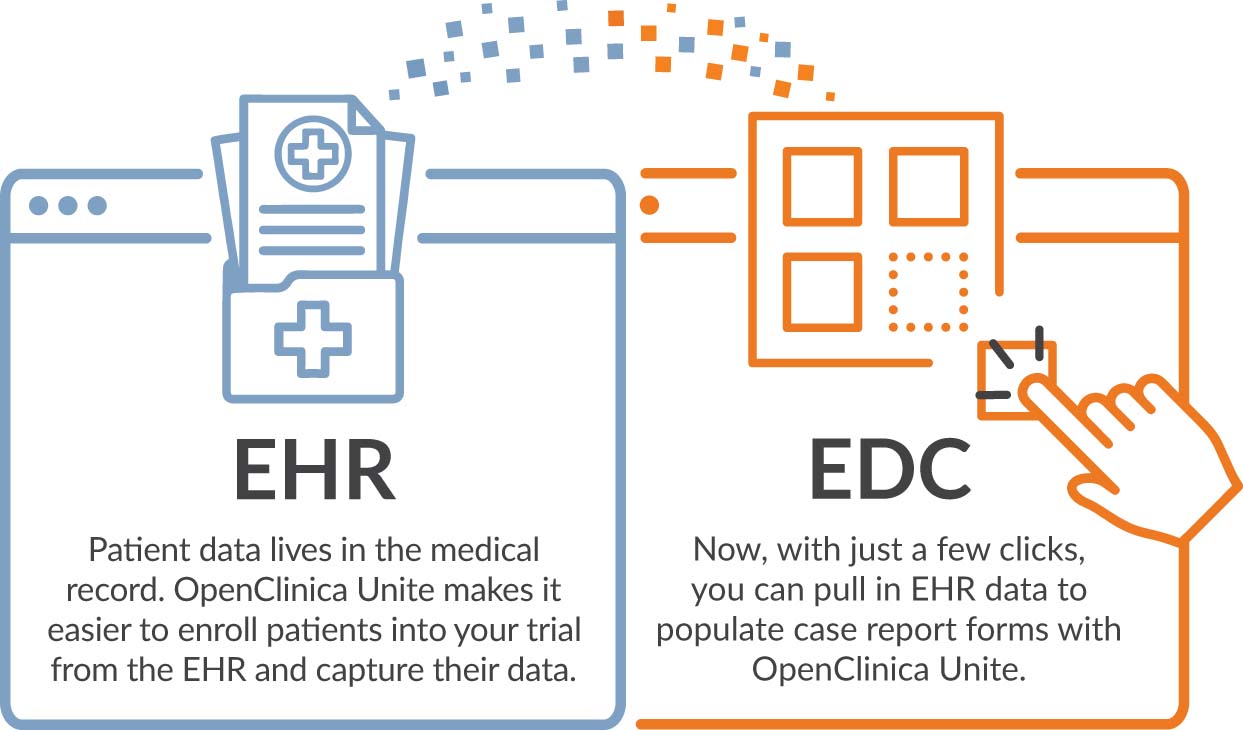
OpenClinica announces the launch of its electronic health record (EHR) eSource solution, OpenClinica Unite. Unite makes it easy for research...
Introducing OpenClinica Unite, your integrated EHR eSource solution
OpenClinica announces the launch of its electronic health record (EHR) eSource solution, OpenClinica Unite. Unite makes it easy for research...
Calculating ROI for ePRO
I recently delivered a webinar titled “Getting Started with eCOA/ePRO,” in which roughly a third of attendees polled cited expense...
Video Demos: Printing Subject Casebooks, Blank Casebooks and Blank CRFs
We’re in the process of updating our documentation to include video demos of various OpenClinica features. Take a look at...